Study: Half Dose of AstraZeneca Just as Effective as Full Dose, but Less Toxic
If correct, this means that a few billion people were needlessly given a higher dose of a product whose toxicities are dose-dependent, in clear violation of the most basic medical ethics.
It seems that every other day now a new major “discovery” is unshackled from the censor ghetto pulverizing pillar after pillar of the mainstream pandemic narrative.
In this spirit, the following study was published August 29 (and was available on the PubMed preprint server from June 10):
Effectiveness, safety, and immunogenicity of half dose ChAdOx1 nCoV-19 COVID-19 Vaccine: Viana project
https://pubmed.ncbi.nlm.nih.gov/36105814/
Basically, the authors wanted to see if a half dose of AstraZeneca provides the same immunological protection that a full dose does. I’m going to give a quick summary here and put the bulk of the relevant language in footnotes.1
The vast, vast majority of studies assessing vaccine safety or efficacy that are retrospective - meaning the authors take data and analyze it to see if they can detect patterns. A major weakness of such studies is that they can be easily confounded by all sorts of other variables and so are usually hard to definitively interpret. Additionally, retrospective studies are far more susceptible to jury-rigging or outright fraud, since researchers can pick datasets to use that are innately biased towards their preferred results. For instance, using US hospital data which systematically categorizes many if not most vaccinated people hospitalized with a positive covid test as unvaccinated will obviously “show” a “pandemic of the unvaccinated”.
This study however is not a retrospective analysis. The researchers here conducted an actual experiment, using two groups, one of which got a full dose of AZ and the other a half dose, for both doses. This allows for a natural experiment to see what happens to each group; in this case, how do the outcomes of a full dose compare to the outcomes of a half dose.
As far as I can tell, this study seems to have been conducted reasonably well enough to provide a solid foundation for its central finding, but there are multiple significant limitations to this study that are important to bear in mind2. Thus although it is not conclusive evidence, it cannot be dismissed nonchalantly as junk science either.
What did they find?
This study compared a half dose to a full dose in three ways:
Covid case rates
Immunological biomarkers
Safety - systemic adverse events (ie the “routine” side effects)
Covid case rates
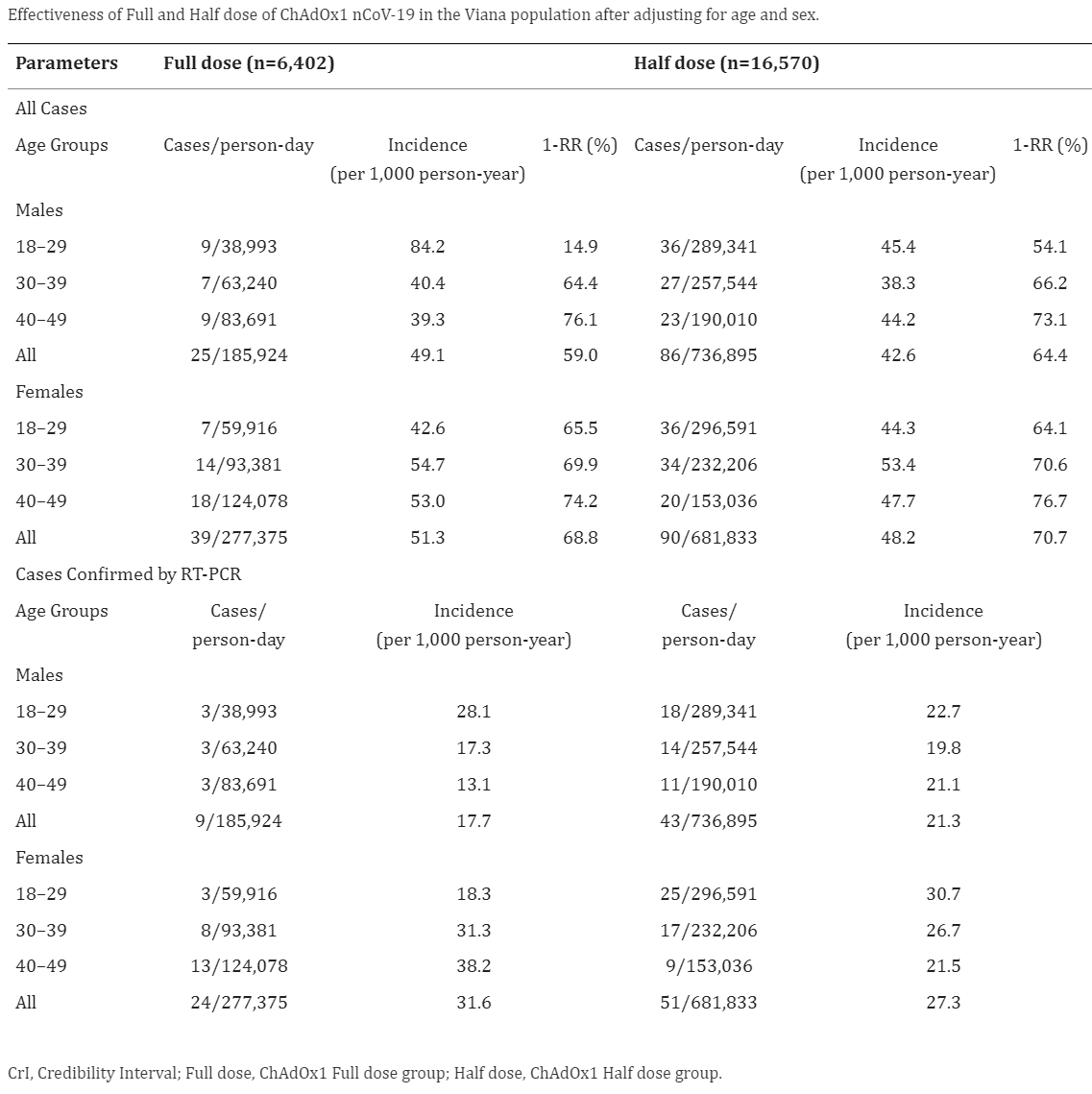
There was no meaningful difference in covid case rates between the half dose and the full dose:
“A half dose of ChAdOx1 nCoV-19 is as effective, safe, and immunogenic as the full dose.”
“Despite this limitation, it was possible to confirm that the effectiveness of half dose is non-inferior to full dose for preventing new cases.”
Immunological biomarkers
This took up the bulk of the study’s length, it is unduly complex, and it is not critical for this article. So I won’t belabor the point except to highlight two quotes from the authors describing the results:
A half dose induced a high increase in plasma chemokines, pro-inflammatory/regulatory cytokines, and growth factors.
The immune response in pre-immune (seropositive in the baseline) individuals indicates that the half dose may be a booster dose schedule.
Safety / Rate of Routine Side Effects
I have reservations about whether they would capture any severe adverse events, but that is another matter.
Regardless, this is what they found:
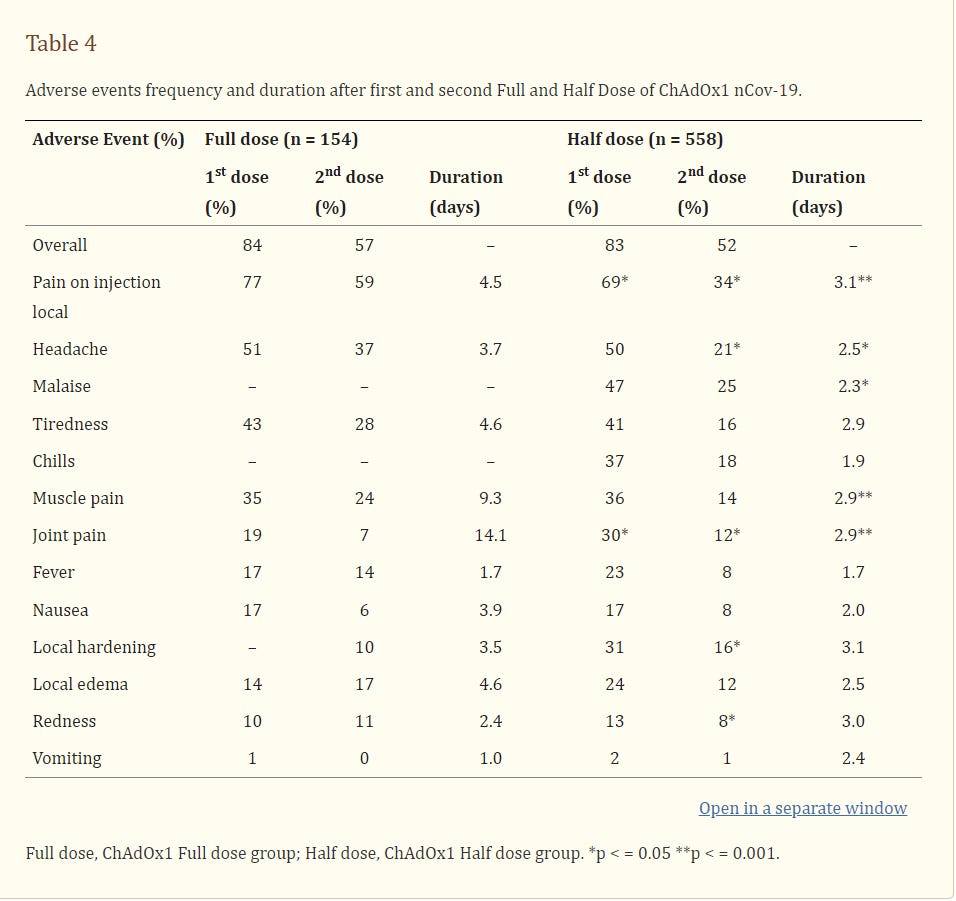
The frequency of adverse events was similar. No serious adverse events or deaths were reported.
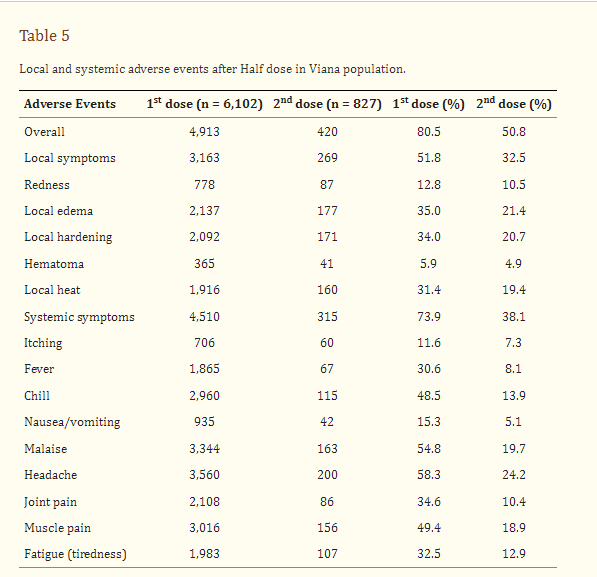
The frequency of AEs was similar in both groups (Table 4), but the duration of symptoms was shorter in ChAd Half Dose group, especially after first dose. No serious AEs, hospitalizations, or deaths were reported in either group. Overall rate of adverse events was 80.5% and 50.8% after first and second dose (Tables 4, 5). Reactogenicity was lower after the second dose in both the ChAd Half Dose and ChAd Full Dose groups (Tables 4, 5).
This study, in a nutshell, found that a half dose confers all of the benefits of the full dose with the same observed immediate routine side effects, albeit for a shorter duration. Or as the authors summed up:
A half dose of ChAdOx1 nCoV-19 is as effective, safe, and immunogenic as the full dose.
Some key takeaways
There are several notable observations that are worth emphasizing.
Dose-Dependent Toxicity✔, Dose-Dependent Benefit❌
It is noteworthy that the side effects occurred at the fairly similar *astoundingly high* rates in both groups. Normal vaccines are not supposed to cause anywhere near this level of systemic reactions.
Critically, there was also a significant difference observed in the duration of these side effects between the half dose and full dose groups. It is thus ethically unconscionable that anyone was given a full dose of AstraZeneca at all - the extra half-dose is per this study all pain and no benefit at all.
The authors themselves broached this point (however tepidly, probably to appease the censors):
For population at more risk for adverse events, low dose could be safer. Ramasany et al. (8) found that a lower dose of vaccine was less reactogenic than the standard dose of vaccine across all age groups. The mixed pattern of immune response, with pro-inflammatory and regulatory biomarkers observed in our study, may be associated with a lower frequency and severity of AEs after half dose. Although it was not possible to confirm this hypothesis with this sample size, this remains a subject of interest for future studies, considering that severe adverse event as thrombocytopenia was rarely reported previously.
Considering the current scenario of having to maintain booster doses regularly to contain hospitalizations and deaths from COVID-19, it is relevant to know that a half dose regimen can confer same effectiveness as a full dose, to protect from infection, hospitalizations, and deaths.
The very notion itself of needlessly administering a medical intervention that had no benefit but increased risk of harm is contrary to the cardinal ethical principle of “first do no harm”.
Un-informed consent
Another subtle yet profound implication of this study, one that goes without saying, is that every single vaccination worldwide was by definition lacking “informed” consent. This is true even if you accept every single other establishment premise about the vaccines. Very few people, even among the most ardent vaccine fanatics, would knowingly and willingly accept “extra” vaccine juice that carried only risk but no benefits. And even for the committed cultists, their consent was nevertheless deficient, because they were not informed.
Failure to conduct elementary pharmacokinetic studies, even after vaccine rollouts
There is no justifiable rationale that this study wasn’t performed on a much larger sample size and for all the vaccines. Any remotely sentient person understands intuitively that we ought to understand the pharmacological properties of a therapeutic so we know how to optimally dose it. This study has multiple not-insignificant limitations that foreclose the possibility of drawing robust conclusions from it alone. The failure of *any* public health authority or agency anywhere in the world to demand such studies be conducted as randomized double-blinded trials and carried out at scale (and not on a mere handful of people handpicked by the pharma company) is simply indefensible.
The ignorance regarding the basic pharmacological properties of the covid vaccines is so blatantly evident at this point that even academics are conceding the obvious3 in the literature:
"Understanding immune responses to SARS-CoV-2 messenger RNA (mRNA) vaccines is of great interest, principally because of the poor knowledge about the mechanisms of protection"
So… “poor knowledge about the mechanisms of protection”, eh? This study was published September 22… **2022**.
The wonders of modern epistemology are quite the marvel indeed. How we can know that that vaccines work so clearly that anyone who so much as expresses the slightest twinge of a doubt is an intellectual luddite, yet have no idea just how the vaccines actually do the protecting, is beyond me, and probably everyone else too.
It seems there is precious little if anything at all that we actually do understand about how these vaccines actually work. Or “work”.
Random fun fact curtesy of Reuters:
“While skill and hard work drove development, AstraZeneca said it was a minor mistake that made the team realise how they could significantly boost the shot’s success rate, to as much as 90% from around 60%: by administering a half dose, followed by a full dose a month later.”
Abstract
Fractional dose is an important strategy to increase access to vaccines. This study evaluated the effectiveness, safety, and immunogenicity of half dose of ChAdOx1 nCoV-19 vaccine. A non-inferiority non-randomized controlled trial compared a half dose of ChAdOx1 nCoV-19 with the full dose, with an interval of 8 to 10 weeks, in individuals aged 18–49 years. The primary endpoints were the incidence rate of new cases/1,000 person-year at 90 days after 14 days of the second dose, confirmed by RT-PCR and new cases registered at SUS National Health Surveillance Database (e-SUS VS). The anti-SARS-CoV-2 spike (S) protein receptor binding domain (RBD) by chemiluminescence and the neutralizing antibodies by plaque reduction neutralization test (PRNT) were titrated. The soluble biomarkers were quantified with a multiplex immunoassay. Follow-up was 90 days after 14 days of the second dose. A total of 29,598 individuals were vaccinated. After exclusion, 16,570 individuals who received half a dose and 6,402 who received full doses were analyzed. The incidence of new cases confirmed by RT-PCR of half dose was non-inferior to full dose (23.7 vs. 25.7 cases per 1,000 persons-year [coefficient group -0.09 CI95%(-0.49 to 0.31)], even after adjusting for age and sex. There were no deaths or hospitalization after immunization of either group. Immunogenicity was evaluated in a subsample (N=558) compared to 154 healthcare workers who received a full dose. The seroconversion rate in seronegative individuals at baseline half dose was 99.8%, similar to that of the full dose (100%). Geometric mean concentration (95% CI; BAU/mL) were half dose = 188 (163-217) and full dose = 529 (423–663) (p < 0.001). In seropositive subjects at baseline (pre-immune individuals), the first dose induced very high and similar IgG-S in half dose 1,359 (1,245-1,483) and full dose 1,354 (1,048–1,749) BAU/mL. A half dose induced a high increase in plasma chemokines, pro-inflammatory/regulatory cytokines, and growth factors. The frequency of adverse events was similar. No serious adverse events or deaths were reported. A half dose of ChAdOx1 nCoV-19 is as effective, safe, and immunogenic as the full dose. The immune response in pre-immune (seropositive in the baseline) individuals indicates that the half dose may be a booster dose schedule.
[Section] 4 Discussion
This large trial evaluated a vaccine against SARS-CoV-2 and confirmed that a half dose of ChAdOx1 nCoV-19 is safe, effective, and immunogenic as the standard full dose. For the overall confirmed cases, the lower bound estimate under a 95% CrI for the effectiveness in the ChAd Half Dose group was within the 95% credibility interval estimated for the ChAd Full Dose group, indicating non-inferiority for this treatment.
A half dose regimen of the COVID-19 vaccine can double the availability of doses and cut costs in half. Considering the current scenario of having to maintain booster doses regularly to contain hospitalizations and deaths from COVID-19, it is relevant to know that a half dose regimen can confer same effectiveness as a full dose, to protect from infection, hospitalizations, and deaths.
The ChAdOx1n19 vaccine is of special interest to Brazil, as it is the vaccine with the lowest cost, about 2-4 times less than other immunizers. Furthermore, it is produced in Brazil, after the transfer of technology from AstraZeneca/Oxford to Fundação Oswaldo Cruz (Fiocruz), which allows for greater production for distribution in Brazil and other countries in Latin America and Africa, as has already occurred in other epidemics.
The study included 88.3% of all individuals of 18-49 years, from Viana City, in Brazil. A previous study showed that a low dose of ChAdOx 1 nCov-19 vaccine induced high titers of neutralizing antibodies, similar to the standard dose, at different ages. It was later shown that a low dose followed by a standard dose conferred greater efficacy than two standard doses (8, 9). Those preliminary studies highlighted the hypothesis that a half dose is immunogenic enough to induce cellular and humoral immune responses and to confer protection against SARS-CoV-2. This is the first study to provide evidence for using fractional doses as a strategy to face COVID-19. Khoury et al. found high correlation between neutralizing antibody response and efficacy against disease (15) to show that half or even quarter doses of some vaccines generate immune responses associated with high vaccine efficacy, as we could demonstrate in this trial.
The main limitations of the study were the lack of randomization and unavailability of a placebo group. The design of the study had to take into consideration the opportunity to perform an interventional trial to study the effectiveness of a half dose of ChAdOx1 when vaccines were available and being distributed in that population.
Despite this limitation, it was possible to confirm that the effectiveness of half dose is non-inferior to full dose for preventing new cases. Effectiveness of ChAdOx1 was estimated around 70% to prevent new cases (9). A retrospective longitudinal study of 75,919,840 Brazilian vaccinees from January to July 2021 evaluated the effectiveness of ChAdOx1 (Vaxzevria) or Coronavac (16). Individuals fully vaccinated (≥14 days after the second dose) with ChAdOx1 had a 78.1% (95% CI: 77.2–79.0) lower risk of developing symptomatic SARS-CoV-2 infection. In the elderly Brazilian population, the effectiveness of the two-dose schedule was 77.9% (95% CI: 69.2–84.2) against COVID-19, 87.6% (95% CI: 78.2–92.9) against hospitalization, and 93.6% (95% CI: 81.9–97.7) against death (17).
There were differences between the ChAd Full Dose and ChAd Half Dose groups according to age and sex distribution; however, the analysis considered random effects and specifically linked the same groups for either vaccinated with a half dose and full dose.
The fact that not all symptomatic cases took the PCR test is another limitation of the study, but we did analysis considering only those cases confirmed by RT-PCR showing non-inferiority between half dose and full dose.
Throughout the study period, the Delta variant was more frequent in most COVID-19 infections, according to the independent genomic surveillance reported in Brazil and Viana. The Delta variant was first identified in June, and by August corresponded with most of the sequences analyzed (data not shown).
We found higher levels of biomarkers after half dose after primary vaccination compared to full dose. We hypothesized that half dose induces later kinetics of biomarkers compared to full dose, and therefore after 28 days, levels were higher in the half dose group. Unfortunately, we did not evaluate the kinetics of biomarkers. Probably, the peak of biomarkers of the full dose was earlier.
This hypothesis can be supported by the observation that the kinetics of biomarkers is earlier in vaccines with a viral vector compared to natural infection with lower levels of viral particles (18).
Beyond advantages based on cost-reduction and production benefits, there is a lower potential of adverse events using fractional half dose. In fact, we reported lower rate and duration of symptoms after half dose. In our study, reported local and systemic AEs were mild, in line with previously reported studies (8, 9, 19). A prospective observational safety study on ChAdOx1 nCoV-19 vaccine in the real-life showed that younger, female, hypertension, history of allergy, and hypothyroidism individuals had two to three times higher odds for adverse events (19). For population at more risk for adverse events, low dose could be safer. Ramasany et al. (8) found that a lower dose of vaccine was less reactogenic than the standard dose of vaccine across all age groups. The mixed pattern of immune response, with pro-inflammatory and regulatory biomarkers observed in our study, may be associated with a lower frequency and severity of AEs after half dose. Although it was not possible to confirm this hypothesis with this sample size, this remains a subject of interest for future studies, considering that severe adverse event as thrombocytopenia was rarely reported previously.
Spike binding and neutralizing antibodies have been proposed as a correlate of protection for COVID-19 vaccines (20). It has been reported that mRNA-1273-vaccinees with PRNT50 titers of 10, 100, and 1000 have estimated vaccine efficacies of 78%, 91%, and 96%, respectively (20). Our findings are in agreement with this proposal as we found a global serum conversion of 100%, considering the PRNT50 titer of 10 as the cut-off and overall effectiveness of 67% and 69% for subjects receiving a full dose or half dose, respectively.
In pre-immune individuals, titers after the second dose were lower than those after the first dose, after a full dose or half dose, suggesting that there may be some mechanism of cellular exhaustion. Further studies are necessary to investigate this phenomenon.
Although there are no defined cellular immunity correlates of protection against COVID-19 infection and the immunological thresholds required for vaccine efficacy remain undefined, a favorable immune profile induced by ChAdOx1 vaccine has been proposed (21). Here, we found that a half dose of ChAdOx1 elicited a strong systemic, polyfunctional, and balanced soluble mediator response. To provide a more detailed overview of SARS-CoV-2-specific cellular response, phenotypic and functional analyses of memory T and B cells are currently under investigation in the Viana study.
A limitation of this study was the use of an external comparison group for immunogenicity assessment, especially due to the predominance of females in the ChAd Full Dose subsample. The significant contribution of sex to modulating vaccine-induced immunity has gained attention over the last several years. Specifically, females typically develop higher antibody responses and experience more AEs following vaccination than males, regardless of age (20, 22). We performed analyzes stratified by sex. The geometric mean titers found were similar in men and women. Comparisons between the ChAd Full Dose and ChAd Half Dose groups reproduced those results found in the general analyses. Despite the significant biological and behavioral differences between males and females, systematic review and meta-analysis concluded no significant sex differences in the efficacy of the COVID-19 vaccines, especially in younger populations (23).
We included the same 18-49 age range for both groups. This is a homogeneous age interval for vaccine reactivity. We conducted additional analysis, and the Spearman correlation of antibody titers (IgG-S) with age was very low (r = 0.1703, p = 0.0192). Our study did not include elderly people because, in that period, that population had already been vaccinated.
Further analyses are required to investigate the usefulness of a half dose for homologous or heterologous boosting as well as the effectiveness in both children and elderly populations. Although laboratory analyses reported in this study were done in blinded assays, ongoing studies of cellular immunity in larger groups will provide relevant additional information about the similarities between half and full doses.
In conclusion, a half dose of ChAdOx1 nCoV-19 is safe, effective, immunogenic, and non-inferior to the full dose. The immune response in pre-immune individuals indicates that the half dose may be a booster dose schedule.
It is important to note that there are several limitations to this study:
The main limitations of the study were the lack of randomization and unavailability of a placebo group. The design of the study had to take into consideration the opportunity to perform an interventional trial to study the effectiveness of a half dose of ChAdOx1 when vaccines were available and being distributed in that population.
The fact that not all symptomatic cases took the PCR test is another limitation of the study, but we did analysis considering only those cases confirmed by RT-PCR showing non-inferiority between half dose and full dose.



Results like this make me think that the product isn't effective at all. It's like the studies showing surgical masks just as effective as n95 respirators. It's because neither work at all.
Which means that NO dosage is the best option, by far.