Is the FDA Sneaking Through a "Future Framework" to Enable a New Direct-To-Consumer Pathway for Novel Pharma Drugs with Unacceptable Risk/Benefit Profiles?
New rule just released by FDA seeks to "broaden the types of drugs that can be approved as nonprescription" by adding a new mechanism for making prescription drugs OTC.
Quick summary
The FDA has proposed a new rule which creates a new category of drugs available OTC without a prescription but that have a risk profile that is too ‘complicated’ to put on the labelling of the pill bottle and therefore aren’t currently eligible for approval as an OTC nonprescription drug.
The relevant question here is not what the FDA’s declared intentions and uses for this are, rather it is what can this new approval mechanism for OTC drugs be used for in the hands of a corrupted government agency like the FDA.
The simple answer seems to be that this is functionally the equivalent of the FDA’s “Future Framework” for new covid vaccines, a way to allow new Pharma products with unacceptable risk/benefit profiles onto the market without adequately testing them and without consumers having an accurate grasp of the risk/benefit profile.
What can possibly go wrong?
*************************************************************
After a chaotic two years, has the FDA finally overcome its culture of Toxic Pharmaninity and discovered the virtues of making “Safe & Effective” TM drugs available over the counter?
That would seem to be the case looking at a brand-new proposed rule just released by the FDA:
I will admit that finding the talking points routinely espoused by frontline doctors such as Pierre Kory, Paul Marik, Peter McCullough and numerous others nestled inside an official FDA announcement is a little… unexpected?
After all, we had been scolded over and over and over again that such fanciful notions of patient autonomy and independent decision making regarding medical interventions were the highest of high heresies. Although these days, you can get severe whiplash trying to follow the daily erratic paroxysms of vacillating up-to-date definitions, approved language and The Current Thing theological catechisms; so you really never know.
In any event, their press release starts off like a PSA from the FLCCC. (Of course not in defense of Ivermectin, HCQ, and a coterie of other therapeutics used by the myriad doctors who treat covid, such as those in the FLCCC’s various protocols.)
I am fully expecting Twitter to deplatform the FDA1 over this. Let’s see2:
People are capable of figuring out what drug to get without direct medical supervision? ✔️
Nonprescription drug products are used by consumers without the supervision of a health care professional and require the ability of the consumer to determine that they have the condition for which the drug is to be used, and to appropriately use the drug.
Patients can be empowered to take better care of themselves if they can access safe drugs over the counter? ✔️
[…] empowering them to self-treat certain common conditions and improving public health
Even if we broaden the range of marketed nonprescription drugs available to consumers? ✔️
[…] this proposal can broaden the types of drugs that can be approved as nonprescription
Using OTC drugs is routine and standard practice? ✔️
[…] millions of people use them to self-manage health conditions every day,” said FDA Commissioner Robert M. Califf, M.D.
All of which have been already adjudicated by the (allegedly) disbanded Truth Ministry’s Council atop the Twittacracy to be fact-checked disinformation.
So has the FDA “seen the light” & genuinely wants to “improve public health” by enabling easier OTC access to cheap & safe drugs?
In a similar vein, is Elvis still alive?
So, what gives?
In all seriousness, the FDA suddenly (re)discovering the fundamental tenets of practicing medicine is brazenly hypocritical and unprincipled.
We can be sure that the FDA would not turnabout to reembrace the Hippocratic Oath unless they saw it as a means to achieve something. And we also know that anything the FDA thinks of as an “achievement” is probably to the detriment of humanity.
So with that in mind, prepare to go for a stroll through the droll drab pages of the Federal Register. A democracy requires an informed citizenry, so I think that it is worthwhile to explore at length parts of this even lengthier proposed rule. I will try to keep it entertaining to make this intellectual waterboarding slightly more palatable (you still might drown though, no guarantees).
If you want a one-sentence summary of the FDA’s copious waste of words:
‘The FDA is claiming it will enforce rigid standards that will definitely prevent any inappropriate gaming of the system or unforeseen consequences from allowing Pharma to flood the market with new OTC drugs marketed directly to consumers to use without any sort of doctor supervision.’
*************************************************************
You might want to open this image in a separate tab. (You can google the terms to get better clarity, this article is almost as long as the rule in the Federal Register already as it is.)
Here is how the FDA describes the purpose of their own rule in the Federal Register - and one thing we have learned from covid is when the bad guys announce their intentions, it behooves us to take them at their word:
Let’s see. There seems to be a bottleneck in creating direct-to-consumer Pharma products because, as it turns out, it’s kind of difficult to squeeze in all the necessary info on a pill bottle the important stuff. You know, like drug-on-drug interactions, side effects to watch out for, contraindications, etc., stuff that is kind of important that a consumer actually read, understand, and internalize before using the drug.
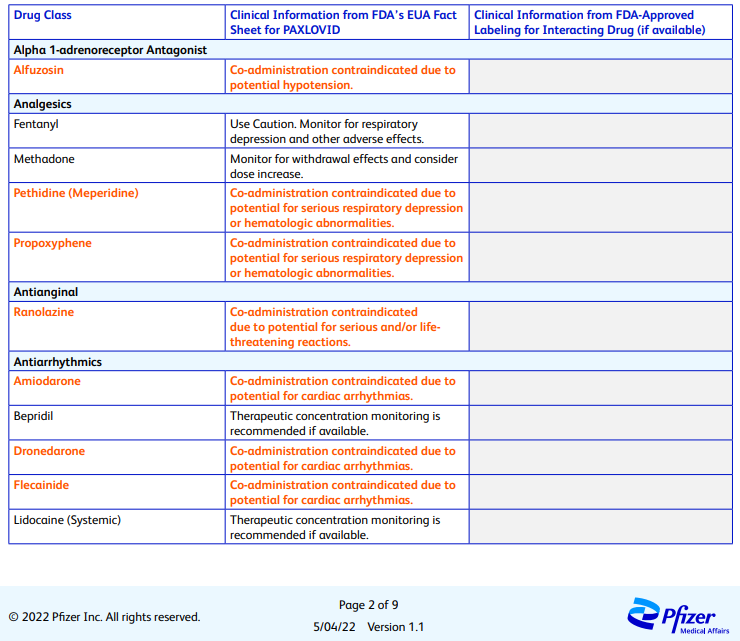
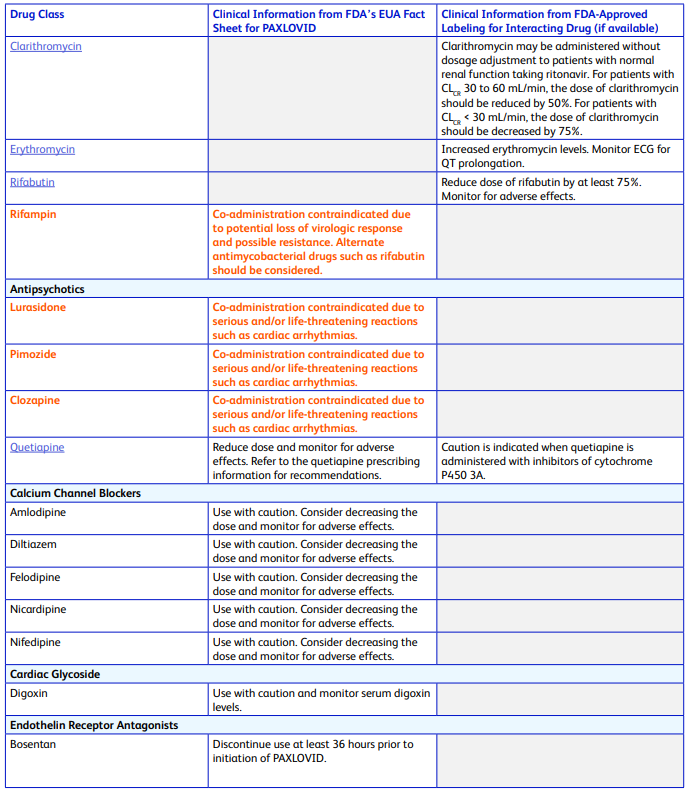
This dynamic presents a wee bit of a problem (to Pharma companies that can see the mounds of cash waiting for them from untold numbers of drugs that have a limited market because they were until now relegated to prescription only). Take Paxlovid for instance - Pfizer’s entire PDF listing all the lovely drug interactions wouldn’t even fit on a box let alone a pill bottle:
There are another 6 pages besides these, and it is written for healthcare professionals. Imagine how long the version of this document written for laypeople might be? You would need multiple Ikea boxes just to fit all the words. That’s a heck of a lot of trees. At least we’d finally solve California’s intractable wildfire problems.
Recap:
The FDA wants to essentially allow for drugs to be OTC even though they can’t really put all the pertinent information (even by the FDA’s own very light standards) somewhere that consumers will reliably access. Remember this as we plunge deeper into the thicket of regulatory mumbo jumbo.
***************************************************************
“The evidentiary standards it must meet”
In other words, yeah, um, what was the efficacy after dose 2 in the Pfizer baby trial again? Exactly.
This is boilerplate language that is basically just saying “we the FDA will create and uphold standards for evidence, safety/efficacy thresholds, etc.
And can we stipulate that “requirements” is a mutually exclusive concept from the FDA (unless it involves the required fees to gain approval that is)?
This charming paragraph is claiming that the FDA will define the types of drugs eligible for this new type of OTC approval so that there actually is a real material difference between drugs that can only be gotten with a prescription and the new breed that will be available OTC conditionally. (Conditions are defined later in the rule.)
But despite the significant material differences, the two super different drug products can have the same:
active ingredient
dosage form
strength
route of administration
indication (ie what it’s used for)
Exhibit A: The significant and clearly discernable material difference between the EUA Pfizer vaccine and the “Approved” Pfizer whoops Comirnaty vaccine.
Go figure.
I honestly am lacking clarity if the FDA actually means anything coherent here beyond “we can pull the same shtick we pulled with the Pfizer vaccine approval where we said putting a different color label and a new trade name on the vials makes it a legally distinct entity despite being the exact same formulation (allegedly).”
😂😂😂😂😂😂😂😂😂😂😂😂😂😂😂😂
This is the same FDA rigorously enforcing the standard of minimum 50% efficacy to be eligible for an EUA. Or at least not having *negative* efficacy. Yeah, me neither.
Or the rigorous and thorough data behind kidney killing Remdesivir that showed a reduction in the primary endpoints stipulated like reduced mortality. Oh wait, it didn’t, they changed the endpoint multiple times midway (also a violation of FDA standards).
🤣🤣🤣🤣🤣🤣🤣🤣🤣🤣🤣🤣🤣🤣🤣🤣
I’ll take ‘postmarketing pharmacovigilance system the FDA ignored’ for $500:
What is…… VAERS???
Other answers include DMED, EudraVigilance, YellowCard, and CMS.
(Contestants are an AI approximate visualization of the FDA personnel who wrote this thing. The AI tried, but as you can see, it was a difficult task to portray such a vigorous level of expertise and scientific excellence.)
And it’s not like Pfizer did a little post marketing of their own…. and promptly hid the results behind the FDA firewall.
*******************************************************
Cost/benefit analysis. Cuz that’s the central tenet of adjudicating the merits of a new drug. Well, it used to be at least. Allegedly.
You gotta love how confident the FDA is that the ACNU “would ENSURE the appropriate self-selection, [] use, or both, of a drug product.” Does any serious person really think that the FDA’s actual standards used will prevent mass “off label” use??? Or straight up Pharma fraud??? Cuz direct marketing to consumers never gave people false impressions about new drugs. Never ever.
And the clincher: the FDA “expects” this would “expand consumer access to ‘certain’ drug products in a nonprescription setting3.”
When the bad guys announce their plans, it’s probably a good idea to take them seriously.
*******************************************************
The following excerpts are from the section titled "I. Executive Summary”:
First observation here is that this is printed on page 38,316 of the Federal Register. Which is still in the first half with quite a ways to go. The Fed Register is the Bible of Bureaucratic Tyranny. Even the people who write these monstrosities have no friggin idea what they wrote afterwards once they’re onto writing the next useless regulation about such crucial things like Standards for Sanitary Toilets in Coal Mines. Cuz without the Feds, who knows what those toilets would look like.
“Nonprescription drug products are important for the treatment of many conditions and disease.”
You know, like COVID.
(Why do I get the feeling like the FDA is planning to allow through drugs to treat vaccine injuries err, LONG COVID. Very very very long covid that would have been much worse without that fifth booster of course. But I digress.)
“Currently, nonprescription drug products are limited to drugs that can be labeled with sufficient information to enable consumers to appropriately self-select and use the drug product without the supervision of a healthcare practitioner.”
“Self-selection is the decision consumers make to use or not to use a drug product based on reading the information on the drug product labeling and applying knowledge of their personal medical history (Ref. 1).”
The FDA seems to be laboring under the deluded notion that people actually look to the stuff on the pill bottle (or on that annoyingly folded sheet stuffed inside the box) to learn about an OTC drug. They all look the same - an endless list of horrible ways you might die but fortunately are so rare that you don’t need to worry about it which then makes you wonder why they would put them on the bottle at all.
I don’t know about you, but I don’t think that gramps can read the tiny letters even with his prescription eyeglasses.
And does the FDA really think that younger people think that pill bottle info is more reliable than TikTok4?? C’mon!!
And lest we forget, because of the debacle of the past two years, now half the country basically lives by the “safe and effective” rule of thumb:
Assume that the opposite of anything the FDA proclaims is true, and ye shall verily prosper.
Alternatively:
Safe & Effective means ☢️☢️☢️ without any doubt whatsoever
The only people who actually read these things are comedians looking for material.
You can maybe - maybe - make a case that some people read some product information on Amazon. Not pill bottle wrappers.
What is… IVERMECTIN.
This could have been written by the FLCCC.
In all seriousness, this is a key paragraph that highlights in blunt terms what the FDA is doing with this rule - to approve drugs that will not have all the pertinent information available. And yes, I am assuming that what will likely happen is that much of the information that ‘doesn’t fit’ on the bottle will end up in the nether regions of the FDA or Pharma company website that nobody will have substantial awareness of. The regulatory scheme likely to emerge is one that can easily allow for highly risky drug products to flood the market without corresponding awareness among the public, especially if Pharma can convince (bribe) doctors to explain to patients how “Safe & Effective” these drugs are.
“The proposed rule has the potential to broaden the types of drug products that FDA could approve as nonprescription.”
BINGO!! Cuz with Pharma products, more is always better.
The rest of this paragraph is basically explaining how a drug that is too risky to be explained in a short blurb - kind of like if the list of potential complications and drug-drug interactions was too lengthy for the speedtalker that takes up half of every drug commercial to say even in a full 30 seconds - can be approved for direct-to-consumer OTC use. Yay!!!
You also gotta love how the FDA repeatedly highlights “chronic conditions” throughout this document, as if there won’t be drugs approved for this novel OTC approval mechanism that can be exploited for plenty of other conditions with a far greater market. Or how we will soon define obesity as a “chronic condition”. Either way.
******************************************************
Onto Subsection B: FDA's Current Regulatory Framework
“includes full reports of investigations to demonstrate that the proposed drug product is safe and effective blah blah blah”
Yeah right.
“Thus, an NDA for a nonprescription drug product must include, among other things, information to demonstrate that consumers can appropriately self-select and use the proposed drug product safely and effectively without the supervision of a healthcare practitioner.”
You know, like this:
And that last bit can’t be right:
“but at least some of the information required for approval comes from studies not conducted by or for the applicant”
Since when has the FDA ever demanded that a trial or study on a new Pharma product be conducted by someone besides the manufacturer?!?!?
Whatevs. Maybe I’m just misreading this and all.
This is regulatory gobbledygook that more or less ins saying in so many words that the FDA can do what they’re doing with the new round of covid vaccines for the now-extinct original Omicron variant - this new version that we want approval for OTC marketing is kind of similar enough to the previous version that it can get approval without jumping through all the hoops again.
“Generally, nonprescription drug products must be labeled with adequate directions for use so the consumer: (1) can use the drug product safely and for the purposes for which it is intended and (2) make an appropriate self-selection decision and appropriately use the nonprescription drug product”
In other words:
(I’m not good at creating memes, hopefully someone can make a better version.)
There's a ton more in this rule, but this hopefully can convey a sense of what this sort of regulatory document is like.
Back in the good old days of the USSR, we could "trust but verify", a luxury that does not apply to the aggressively evil administrative state.
Now, it's trust all right, trust that they want to destroy you. No need to verify.
Ok. Not the FDA. Just anyone else who *quotes* the FDA.
Well, there is this delusional bit of entertaining nonsense:
Consumers are likely familiar with the “Drug Facts Labeling” on nonprescription drug packaging as the primary source for information about a product’s intended use, directions for use, and important safety information, all designed in understandable language that is tested for consumer comprehension
I truly pity any poor bloke whose primary source of drug information is the pill bottle. Drug information is communicated about as clearly as wine characteristics on the wine bottle, except that with wine bottle poetry, people at least can follow the words even if they can’t quite be certain that they can taste what the vintner vernacular claims is there. (What on earth is “hints of forest floor” (pun intended) or “edgy flavor of pencil shavings”?? PENCIL SHAVINGS????).
Why the sudden loss of confidence? The FDA doesn’t merely “expect” that it’s standards WILL ENSURE no inappropriate gaming the system.
Or that they can read in the first place if they reside in certain jurisdictions where the literacy rates of high school *graduates* is… well… ‘uninspiring’





















Why not? They have been doing this under the radar for decades. Since there are no penalties for big pharma, the FDA and CDC or AMA murdering people with drugs, vaccines and now mRNA gene therapy injections, it's full speed ahead.
So many people demand drugs, are hooked on their pills and trust big pharma and their doctors without question...the stage is set for ramming new drugs onto the market without trials (which are mostly fake anyway) and charging astronomical prices which the stupid government and health care operations gladly pay.
The 30% who take no drugs are on their own. Should any of those taking drugs, vaccines and mRNA injections be murdered, rest in peace knowing the culprits will never be found or charged with anything.
All OTC REFLUX DRUGS, ALLERGY, INHALERS, have unreadable labels and once were Script drugs. If you don't know what they do, or control you could be in trouble. Not a word if they contain steroids or cortisones. Which Diabetics and Glaucoma patients aren't allowed. Your doctor may fail to tell you about, and if you see several, bad things can happen.
My Gastro informed me the OTC brand of NEXIUM only contained 10 mg of the main drug, label states 20 mg, false advertisement. I was on 40 mg Script, till Tricare cut it. He failed to read my reaction list, or thought I wouldn't know what the generic Protonic is for...Prilosec, was he trying to fool me? It's is on my reaction list, he probably didn't read. As I have Barrett's Esophagus a pre-cancer, I have to have the right amount.
I'm a label reader out of necessity. Foods that contain 'Additives' that are natural, thus don't have to be named, a natural product is Carrageen is a seaweed, it is as bad as OA drugs, or all those pain meds that are GI tract destroyers.
Ask why babies are now on Nexium, Carrageen is a Thickener/Emulsifier it is in most formulas, dairy, pudding, jelly beans, your toothpaste, even organic food. Which the true Organic eaters have filed lawsuits. It is listed as Additive. Allowed to by the FDA. EVERY DRUG THEY APPROVED AT ONE TIME HAS BEEN RECALLED. METFORMIN needs to join the list. Ask Robert Yoho, or read his book Butchered by Healthcare, government and pharma. Best $5 spent on a Kindle book. It will set the Stage for RFK JR FAUCI, just ignore his inbred Democract bias, both parties take Pharma's bribes.
Same could be said of supplements. CDC issued a warning on GUMMY BEAR Melatonin, as more people are using it. This is a decade old issue, Daycare, Pre-K, Kindergarten teachers were giving it to Your kids. 2 infants died, 3 months and 12 months, thousands were sent to the ER, or hospitalized. Those caught, the legal system dealt with. It is the availability of Adult Gummy Bear supplements OTC. more are on the shelves than the pill form now.
I don't advocate removing them, just use Child proof caps small kids can't open, many are dangerous to small children in Adult doses. What we know as Bone supplements, Calcium and Magnesium, are also heart regulators. A unknown Type 1 Diabetic small child eating to many C 500 mg could be sent into Hyperglycemia, which is very serious, or deadly. Not all recall the Tylenol Poisonings case that forced the Childproof caps in the first place. Laundry, dishwasher soap attracted small kids to eat them. They are just now putting childproof capes on them. The quality varies, some won't reseal as childproof. Tide, or the higher end ones, look more promising. Since I don't buy them, they are an expensive gimmick, that is no better than regular style that cost less, has more loads.
I take 40 mg Lutein for Dry Eye Syndrome on the Cornea doctor's advice, since Kroger's bought out Vitacost, it is harder to find, Amazon kept taking me back to those ARERDS, 10 mg Lutein, 500 mg C, 180 E, 9 Zinc, Omega 3 from Fish, no type given. 1) I'm allergic to seafood, you didn't state the source for fish, most are seafood. 2) 10 mg? 3) I'm Diabetic, C runs your BS up, it is why most Citrus fruits are limited. It is not safe for me to take. I have to read labels, as even food impacts my health.
With the right supplements which both Earl Mendel and Linus Pauling had great advice, you can improve your health. You just have to be versed in what they do, and be patient. Same goes for prescription drugs, don't rely on your doctor, look up everyone you are prescribed, before you take them. OP drugs destroy your jaw bone, or can kill. As a Senior, I want to take care of my health, but not with some costly, side effect riddled, never should have been approve drug.
Nor do I want my G. Grands, to suffer what my Granddaughter did with the Useless Gardasil shot that doesn't work to prevent cervical cancer, but has horrid side effects. She nearly died, was left with Rheumatoid Arthritis and Fibromyalgia, both life long painful conditions. She was an active Ribbon Gymnast, multiple sports, now not very active. Wasn't even sexually active, her mom kept her to busy in sports and Christian activities. Ask the poor kids Gates crippled with it, or killed in India. Or the Polio shots that were HIV laced in Africa. Same as the Covid shots were.