42 Case Reports of Myocarditis that were Submitted to Pre-Print Servers and/or Journals BEFORE July 1st... in 2021
In other words, well before the CDC/FDA openly admitted that the vaccines were causing myocarditis
As anyone reading this is doubtless aware, the FDA initially denied the link between myocarditis and the covid vaccines; in this case, ‘initially’ means to say for the better part of 2021. Even now, they are continuing to double down on false and frivolous assertions about vaccine-associated myocarditis, such as that it’s less frequent and less severe than myocarditis caused by covid infection.
Some apologists are now trying to rewrite the history of the medical community’s stance on this issue, claiming that health officials acknowledged that the covid vaccines were causing myocarditis (however rare and mild) immediately following the emergence of indications of a potential association between the covid vaccines and increased incidence of myocarditis.
When did they first acknowledge the *possibility* that the vaccines *might* be causing myocarditis?
As far as I’m aware, the first official acknowledgement of the possibility of vaccine-associated myocarditis came at the end of May:

The FDA here was trying to downplay the possibility that myocarditis was connected to the vaccines, with FDA vaccine chief Dr. Peter Marks saying he would vaccinate his own kids. Not exactly an honest disclosure that could conceivably be considered to fulfill the basic requirements of “informed consent”.
(Side observation: Someone injected a child on *May 19, 2021*.)
It wasn’t until July 6 that the CDC posted a MMWR report - “Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices — United States, June 2021” - acknowledging that myocarditis was indeed confirmed in at least a few instances as caused by one of the covid vaccines.1 (Keep in mind that even after this MMWR was released, the medical establishment continued to either deny or downplay vaccine myocarditis for months in the public communications and messaging, so even this ‘admission’ was ‘lacking’, to say the least.)
This MMWR report covered the June 23 ACIP meeting where data concerning myocarditis (among other potentially vaccine-related adverse events) from the various pharmacovigilance systems was presented.
Notably missing however was any mention of the case studies reporting vaccine associated myocarditis, despite the submission of at least 36 case reports documenting *145* cases of vaccine-associated myocarditis before June 23.
Although this might seem like a trivial number compared to the 1,000+ VAERS reports of myocarditis that were submitted during that time, it is not.
Firstly, the medical establishment’s (absurd) claim that VAERS reports are uniformly unreliable because (allegedly) any random person could submit one does not apply to case reports, which are formal academic literature written by trained clinicians documenting expert clinical opinion and experience.
Secondly - and more importantly - there are at least 11 case reports documenting a minimum of 4 separate cases of presumed vaccine myocarditis, that presented to ONE medical facility, typically within at most 4-7 days of vaccination, over a relatively short timespan (anywhere from 4-12 weeks), with robust exclusions (usually they exclude anyone with past history of either covid infection or myocarditis). This includes one case series that documented 8 cases, and one that documented 13 - THIRTEEN (!!) - despite all of the abovementioned limitations. The odds of having so many cases even with such widely ranging and encompassing exclusions if the vaccines were not responsible is so infinitesimally small as to be a joke.
NOTE:
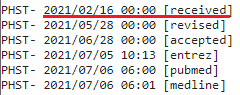
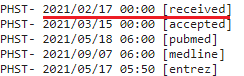
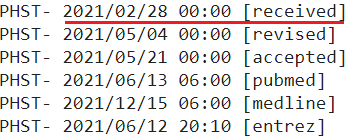
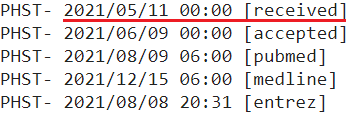
I chose to use the date a study was submitted and not the date it was first published online because the government agencies and public health officials responsible for vaccine policies and determinations had access to studies that were submitted, and can thus not claim to have been “unaware” that they existed.
I used the PubMed metadata page if I couldn’t find the date received on the study’s regular page, which looks like this:
I included both the PubMed metadata & the regular study page for the first few studies so you can see they’re the same. Additionally, all PubMed screenshots are linked to the source, so you can check for yourself that they are accurate.
In the few instances where there is no date provided for the study’s submission, I used the next earliest date available (either the date accepted or the date published online).
The studies are organized chronologically starting from oldest/earliest.
The following 42 case reports documenting peri/myocarditis or myocardial injury were all submitted before July 1, 2021:
1. Acute myocarditis associated with anti-COVID-19 vaccination (Nevet)
https://pubmed.ncbi.nlm.nih.gov/34222133/
Cases: 3
We report on three young male patients, who developed acute myocarditis 2 days after receiving the second dose of the BNT162b2 vaccine.
2. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19 (García et al)
https://pubmed.ncbi.nlm.nih.gov/33994339/
Cases: 1
Given the temporal association between vaccination and the onset of symptoms, and having excluded other acute cardiological disorders, we suggest that this acute myocarditis was an adverse reaction to the BNT162b2 vaccine.
3. Myocarditis After SARS-CoV-2 Vaccination: A Vaccine-Induced Reaction? (D’Angelo et al)
https://pubmed.ncbi.nlm.nih.gov/34118375/
Cases: 1
We report a case of a 30-year-old man who presented progressive dyspnea and constrictive retrosternal pain after receiving SARS-CoV-2 vaccine. Cardiac magnetic resonance and laboratory data revealed typical findings of acute myopericarditis.
4. Myocarditis following COVID-19 mRNA vaccination (Abu Mouch et al)
https://pubmed.ncbi.nlm.nih.gov/34092429/
Cases: 6
We report six cases of myocarditis, which occurred shortly after BNT162b2 vaccination.
5. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection (Ammirati et al)
https://pubmed.ncbi.nlm.nih.gov/33821210/
Cases: 1
An otherwise healthy 56-year-old man presented to the emergency department complaining of acute onset of chest pain 3 days after the second dose of BNT162b2 mRNA COVID-19 vaccine.
6. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases (Mansour et al)
https://pubmed.ncbi.nlm.nih.gov/34166884/
Cases: 2
We report two cases of myocarditis, in two young and previously healthy individuals, temporally related to the second dose of the mRNA-COVID-19 vaccine.
7. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military (Montgomery et al)
https://pubmed.ncbi.nlm.nih.gov/34185045/
Cases: 23
This retrospective case series studied patients within the US Military Health System who experienced myocarditis after COVID-19 vaccination between January and April 2021. Patients who sought care for chest pain following COVID-19 vaccination and were subsequently diagnosed with clinical myocarditis were included.
8. Acute Myocardial Injury Following COVID-19 Vaccination: A Case Report and Review of Current Evidence from Vaccine Adverse Events Reporting System Database (Deb et al)
https://pubmed.ncbi.nlm.nih.gov/34219532/
Cases: 1
Here we present a case of acute myocardial injury that developed as a result of an acute immune response following the second dose of COVID-19 vaccination (Moderna) in a 67-year-old man who presented in acute congestive heart failure.
9. A case report: symptomatic pericarditis post-COVID-19 vaccination (Ashaari et al)
https://pubmed.ncbi.nlm.nih.gov/34693198/
Cases: 1
We described a case of pericarditis post-first dose of Pfizer-BioNTech vaccine.
10. Myocarditis following COVID-19 vaccination (Albert et al)
https://www.sciencedirect.com/science/article/pii/S1930043321003289
Cases: 1
We report a case of a 24-year-old man who presented to the hospital with acute substernal chest pain, 4 days after his second COVID-19 Moderna vaccination.
11. Myocarditis and pericarditis after vaccination for COVID-19 (Hudson et al)
https://pubmed.ncbi.nlm.nih.gov/34337595/
Cases: 2
Two previously healthy males presented to the emergency symptoms with signs of pericarditis/myocarditis after being vaccinated with an mRNA vaccine for COVID-19.
12. Pericarditis after administration of the BNT162b2 mRNA COVID-19 vaccine (Ramírez-García et al)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8196309/
Cases: 2
Here we report 2 cases of pericarditis after administration of the BNT162b2 vaccine.
13. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination (Kim et al)
https://pubmed.ncbi.nlm.nih.gov/34185046/
Cases: 4
Objective: To describe a group of 7 patients with acute myocarditis over 3 months, 4 of whom had recent messenger RNA (mRNA) COVID-19 vaccination.
14. Acute myocarditis following administration of BNT162b2 vaccine (Habib et al)
https://pubmed.ncbi.nlm.nih.gov/34189042/
Cases: 1
Here, we describe a young male patient, who presented with acute myocarditis, three days after the second dose of BNT162b2 vaccine.
15. Possible Association Between COVID-19 Vaccine and Myocarditis (Shaw et al)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8245050/
Cases: 4
We present cardiac magnetic resonance (CMR) imaging findings in 4 cases of acute myocarditis that were temporally related to the receipt of COVID-19 vaccine and could raise the possibility of being associated with the vaccination (Figures 1, ,2, ,3, 4, and and 5 ).
16. Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report (Hasnie et al)
https://pubmed.ncbi.nlm.nih.gov/34348657/
Cases: 1
17. Transient Cardiac Injury in Adolescents Receiving the BNT162b2 mRNA COVID-19 Vaccine (Snapiri et al)
https://pubmed.ncbi.nlm.nih.gov/34077949/
Cases: 7
This is a case series of patients diagnosed with perimyocarditis following vaccination.
18. A spectrum of cardiac manifestations post Pfizer-BioNTech COVID-19 vaccination (Lee et al)
https://academic.oup.com/qjmed/article/114/9/661/6311674
Cases: 3
In this case series, we describe three previously asymptomatic patients who presented with cardiac-related manifestations shortly after receiving the Pfizer-BioNTech COVID-19 vaccine.
19. Case report: acute myocarditis following the second dose of mRNA-1273 SARS-CoV-2 vaccine (Tailor et al)
https://pubmed.ncbi.nlm.nih.gov/34514306/
Cases: 1
A 44-year-old man presented with chest pain and ST-segment elevation 4 days after receiving the second dose of mRNA-1273 SARS-CoV-2 Vaccine.
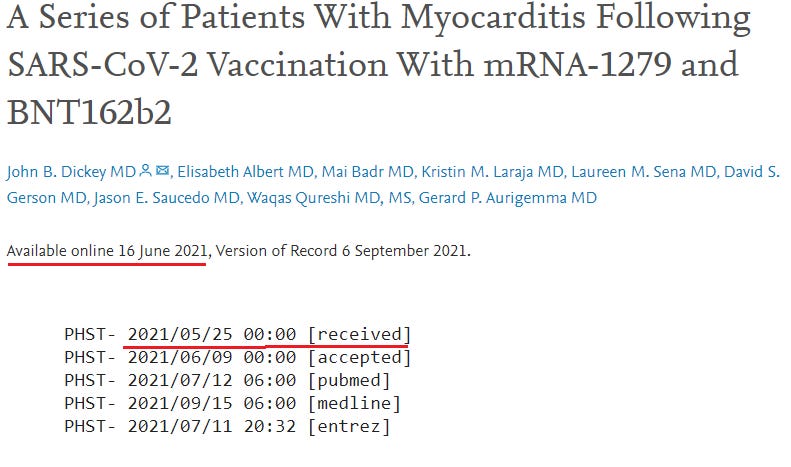
20. A Series of Patients With Myocarditis Following SARS-CoV-2 Vaccination With mRNA-1279 and BNT162b2 (Dickey et al)
https://www.sciencedirect.com/science/article/pii/S1936878X21004861
Cases: 6
Six previously healthy men (17-37 years of age) with no infectious prodrome developed severe chest pain and elevated troponin I within 2 days-4 days of their second vaccination (Figure 1).
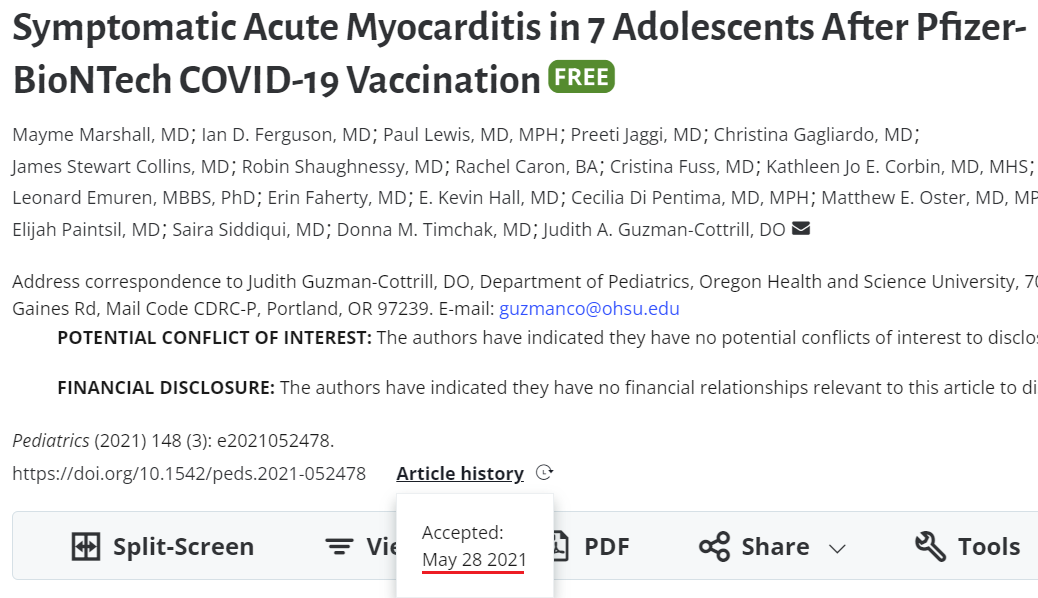
21. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination (Marshall et al)
https://pubmed.ncbi.nlm.nih.gov/34088762/
Cases: 7
We report 7 cases of acute myocarditis or myopericarditis in healthy male adolescents who presented with chest pain all within 4 days after the second dose of Pfizer-BioNTech COVID-19 vaccination.
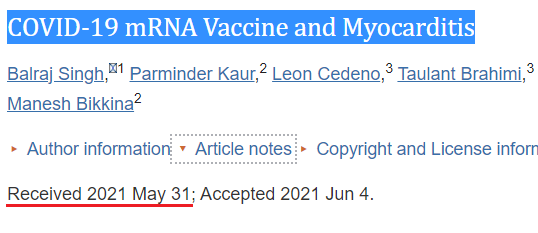
22. COVID-19 mRNA Vaccine and Myocarditis (Singh et al)
https://pubmed.ncbi.nlm.nih.gov/34268277/
Cases: 1
We report the case of 24-year-old male patient who developed chest pain after administration of the second dose of the Pfizer-BioNTech mRNA COVID-19 vaccine and who was diagnosed with myocarditis on work-up.
23. Perimyocarditis in Adolescents After Pfizer-BioNTech COVID-19 Vaccine (Tano et al)
https://academic.oup.com/jpids/article/10/10/962/6329543
Cases: 8
We describe clinical characteristics of 8 adolescents who presented over the course of 36 days to Nicklaus Children’s Hospital with perimyocarditis within 4 days of receiving a dose of BNT162b2 vaccine.
24. Myocarditis after BNT162b2 vaccination in a healthy male (Watkins et al)
https://pubmed.ncbi.nlm.nih.gov/34229940/
Cases: 1
In this article, we present a case of a 20-year-old male with no prior medical history who presented to the emergency department (ED) with chest pain. He had received the BNT162b2 vaccine two days prior to his presentation to the ED.
25. Myocarditis Associated with mRNA COVID-19 Vaccination (Starekova et al)
https://pubmed.ncbi.nlm.nih.gov/34282971/
Cases: 5
Five patients (4:1 male:female; age range, 17–38 years) were identified who had abnormal MRI findings and were vaccinated against COVID-19 prior to MRI.
26. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: A case report (McLean & Johnson)
https://pubmed.ncbi.nlm.nih.gov/34133825/
Cases: 1
We report the case of a previously healthy 16‐year‐old male who developed myopericarditis following the second dose of his Pfizer‐BioNTech COVID‐19 vaccine, with no other identified triggers.
27. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID-19 vaccination: a case series (Patel et al)
https://pubmed.ncbi.nlm.nih.gov/34496880/
Cases: 5
We report a case series of 5 young male patients with cardiovascular magnetic resonance (CMR)-confirmed acute myocarditis within 72 h after receiving a dose of an mRNA-based COVID-19 vaccine.
28. Myocarditis and Other Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines (Vidula et al)
https://pubmed.ncbi.nlm.nih.gov/34277198/
Cases: 5
In this case series, we describe two patients with clinically suspected myocarditis, one patient with stress cardiomyopathy, and two patients with pericarditis after receiving an mRNA-based COVID-19 vaccine.
29. Myocarditis following COVID-19 vaccination - A case series (Levin et al)
https://pubmed.ncbi.nlm.nih.gov/34535317/
Cases: 8
We identified 7 cases of myocarditis with symptoms starting in the first week after the second dose of COVID-19 Pfizer-BioNTech vaccine.
30. Myocarditis After BNT162b2 and mRNA-1273 Vaccination (Larson et al)
https://pubmed.ncbi.nlm.nih.gov/34133884/
Cases: 8
Here, we describe 8 patients who were hospitalized with chest pain and who were diagnosed with myocarditis by laboratory and cardiac magnetic resonance imaging within 2 to 4 days of receiving either the BNT162b2 or mRNA-1273 vaccine (Table).
31. In-Depth Evaluation of a Case of Presumed Myocarditis After the Second Dose of COVID-19 mRNA Vaccine (Muthukumar et al)
https://pubmed.ncbi.nlm.nih.gov/34133883/
Cases: 1
A 52-year-old man presented to the emergency department ≈90 min after the onset of substernal chest pain. Three days before presentation, he received his second dose of mRNA-1273 (Moderna) vaccine for coronavirus disease 2019 (COVID-19), and the next day had a severe reaction that he described as being the “worst he had ever felt.”
32. Myocarditis Temporally Associated With COVID-19 Vaccination (Rosner et al)
https://pubmed.ncbi.nlm.nih.gov/34133885/
Cases: 7
We present a case series of 7 patients hospitalized for acute myocarditis-like illness after COVID-19 vaccination, from 2 US medical centers in Falls Church, VA, and Dallas, TX.
33. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients (Abbate et al)
https://pubmed.ncbi.nlm.nih.gov/34416319/
Cases: 2
We herein report two cases of fulminant myocarditis following BNT162b2 mRNA Covid-19 vaccination associated with systemic hyperinflammatory syndrome and refractory shock requiring support with veno-arterial extracorporeal membrane oxygenation.
34. Acute Myocarditis Following mRNA-1273 SARS-CoV-2 Vaccination (Williams et al)
https://pubmed.ncbi.nlm.nih.gov/34308326/
Cases: 1
In this report, we present a case of acute perimyocarditis in a young, healthy man after vaccination with the mRNA-1273 severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2; Moderna) vaccine.
35. Myopericarditis After the Pfizer Messenger Ribonucleic Acid Coronavirus Disease Vaccine in Adolescents (Schauer et al)
https://pubmed.ncbi.nlm.nih.gov/34228985/
Cases: 13
We describe 13 patients aged 12-17 years who presented with chest pain within 1 week after their second dose of the Pfizer vaccine and were found to have elevated serum troponin levels and evidence of myopericarditis.
36. Takotsubo syndrome after receiving the COVID-19 vaccine (Fearon et al)
https://pubmed.ncbi.nlm.nih.gov/34539938/
Cases: 1
This case presents a post-menopausal woman with a recent diagnosis of MINOCA who developed TTS shortly after receiving the COVID-19 vaccine.
37. Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis? (Chamling et al)
https://pubmed.ncbi.nlm.nih.gov/34333695/
Cases: 3
We would like to present the findings of three different patients that presented to our hospital until mid of June 2021 and showed unusual serious adverse cardiovascular events of infarct-like myocarditis (in the absence of CAD), possibly linked to preceding COVID-19 vaccination. CMR imaging revealed some interesting patterns of myocardial damage suggestive of “autoimmune” myocarditis that show some minor differences to the well-known CMR pattern of “viral” myocarditis.
38. Acute Myocarditis after COVID-19 vaccination: A case report (Schmitt et al)
https://pubmed.ncbi.nlm.nih.gov/34740463/
Cases: 1
A 19-year-old man, presenting with troponin-positive acute chest pain, was referred to our department. He had received the Pfizer-BioNTech COVID-19 vaccine three days prior to his admission. The diagnosis of acute myocarditis was confirmed by cardiovascular magnetic resonance imaging.
39. Self-limited myocarditis presenting with chest pain and ST segment elevation in adolescents after vaccination with the BNT162b2 mRNA vaccine (Park et al)
https://pubmed.ncbi.nlm.nih.gov/34180390/
Cases: 2
Two adolescent males presented within 3 days after the first and second dose of the BNT162b2 vaccine with chest pain. Elevated troponin levels, ST segment elevation, and enhancement of the myocardium in cardiac MRI suggested myocarditis.
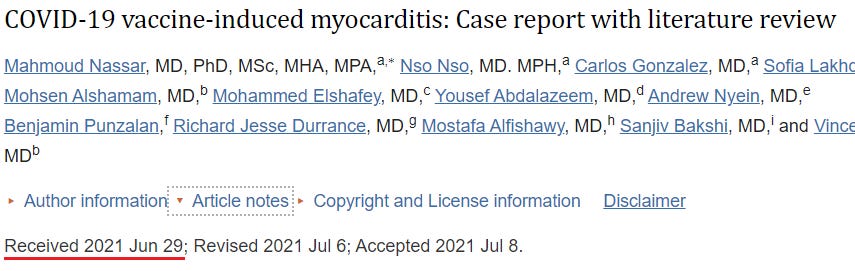
40. COVID-19 vaccine-induced myocarditis: Case report with literature review (Nassar et al)
https://pubmed.ncbi.nlm.nih.gov/34293552/
Cases: 1
A 70-year-old Caucasian female with a history of multiple sclerosis presented to the hospital after two days of receiving the Janssen COVID-19 vaccine.
41. A Case of Acute Pericarditis After COVID-19 Vaccination (Sonaglioni et al)
https://pubmed.ncbi.nlm.nih.gov/35387019/
Cases: 1
Herein we describe the case of a middle-aged healthy women who developed symptoms and signs of acute pericarditis 7-10 days after the second dose of Pfizer-BioNTech COVID-19 vaccination.
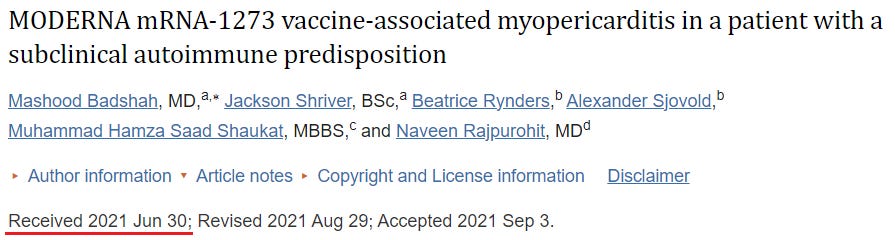
42. MODERNA mRNA-1273 vaccine-associated myopericarditis in a patient with a subclinical autoimmune predisposition (Badshah et al)
https://pubmed.ncbi.nlm.nih.gov/34868402/
Cases: 1
We present a rare case of myopericarditis developing one day after the injection of the second dose of the MODERNA mRNA-1273 vaccine.
On June 14, the BMJ published a letter to the editor titled “Myocarditis followed by CoViD-19 vaccines: A cause for concern or a reversible minor effect?”, which unsurprisingly failed to gain any traction.








































Ashmedai, this is VERY important work you have done (all your work is). I'm printing it out, for my doctors, and others - this is what the lawyers need, too. I'm sending a hard copy to Renz' office (just in case), the lawyers on my list, state, agency, local contacts. This is the work needed for claims and criminal filings. They are on notice, NIAID, NIH, CDC, FDA etc. they are all on notice from several sources. My local health district too. And the medical directors of the hospital clinics. Lots of postage tomorrow!!! Thank you.
And this is just the tip of the iceberg because many were killed by severe myocarditis induced cardiac arrest before they could even be diagnosed. Pfizer's own trial showed 4X cardiac arrest death rates in the vaccinated.
https://vinuarumugham.substack.com/p/to-prevent-one-covid-19-related-death
And Dr. Patrick Whelan of UCLA warned the FDA BEFORE EUA about this specific problem.
https://www.regulations.gov/document/FDA-2020-N-1898-0246
"it is important to assess in vaccinated subjects the effects of vaccination on the heart"