49 Case Reports Documenting *Fatal* Vaccine Associated Adverse Events
Well at least they were transient even if they weren't exactly 'mild'
This needs no introduction.
Enjoy!!
Format:
Main article will contain the title, lead author, link, vaccine/dose, and # of patients for each case report study. Excerpts from each describing the basic clinical events are in endnotes.
Note #1: All case reports here pertain to only the “Big 4” quadruplet (Pfizer, Moderna, J&J, AstraZeneca).
Note #2: There are a few case reports here that are a bit ‘shaky’, insofar as attribution of the death to the vaccine. Not every single case report documenting an adverse event following vaccination necessarily represents an adverse event *caused* by the vaccine (see Introduction to Vaccine Injury Case Reports for more details).
Category #1: Autopsies
The following case reports are autopsies performed on patients who died following an adverse event associated with one of the covid vaccines:
Autopsy #1: Post-mortem findings in vaccine-induced thrombotic thombocytopenia1 (Pomara et al)
https://haematologica.org/article/view/haematol.2021.279075
2 patients, both AstraZeneca, dose unspecified (implied both Dose #1)
Autopsy #2: An autopsy case report of fulminant myocarditis: Following mRNA COVID-19 vaccination2 (Hoshino et al)
https://pubmed.ncbi.nlm.nih.gov/35812802/
1 patient, Moderna Dose #1
Autopsy #3: An autopsy case of fulminant myocarditis after severe acute respiratory syndrome coronavirus 2 vaccine inoculation3 (Satomi et al)
https://pubmed.ncbi.nlm.nih.gov/36040128/
1 patient, Pfizer Dose #1
Autopsy #4: Histopathologically TMA-like distribution of multiple organ thromboses following the initial dose of the BNT162b2 mRNA vaccine (Comirnaty, Pfizer/BioNTech): an autopsy case report4 (Kaimori et al)
https://pubmed.ncbi.nlm.nih.gov/36203145/
1 patient, Pfizer Dose #1
Autopsy #5: A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against Covid-195 (Mörz)
https://www.preprints.org/manuscript/202206.0308/v2
1 patient, Pfizer Dose #3
Autopsy #6: Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings6 (Choi et al)
https://pubmed.ncbi.nlm.nih.gov/34664804/
Autopsy #7: Autopsy Histopathologic Cardiac Findings in 2 Adolescents Following the Second COVID-19 Vaccine Dose (Gill et al)7
2 patients, both Pfizer Dose #2
Autopsy #8: Histological and immunohistochemical findings in a fatal case of thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination8 (Pomara et al [#2])
https://pubmed.ncbi.nlm.nih.gov/35144085/
1 patient, AstraZeneca Dose #1
Autopsy #9: First Identified Case of Fatal Fulminant Necrotizing Eosinophilic Myocarditis Following the Initial Dose of the Pfizer-BioNTech mRNA COVID-19 Vaccine (BNT162b2, Comirnaty): an Extremely Rare Idiosyncratic Hypersensitivity Reaction (Ameratunga et al)9
https://pubmed.ncbi.nlm.nih.gov/34978002/
1 patient, Pfizer Dose #1
Autopsy #10: Vaccine-induced severe thrombotic thrombocytopenia following COVID-19 vaccination: a report of an autoptic case and review of the literature (Fanni et al)10
https://pubmed.ncbi.nlm.nih.gov/34355379/
1 patient, AstraZeneca Dose #1
Autopsy #11: Deterioration of vaccine-induced immune thrombotic thrombocytopenia treated by heparin and platelet transfusion: Insight from functional cytometry and serotonin release assay (Bérezné et al)11
https://pubmed.ncbi.nlm.nih.gov/34485807/
1 patient, AstraZeneca Dose #1
Autopsy #12: Fatal thrombotic microangiopathy with rhabdomyolysis as an initial symptom after the first dose of mRNA-1273 vaccine: A case report (Kamura et al)12
https://pubmed.ncbi.nlm.nih.gov/35189339/
1 patient, Moderna Dose #1
Autopsy #13: Fatal Systemic Capillary Leak Syndrome after SARS-CoV-2Vaccination in Patient with Multiple Myeloma (Choi GJ et al)13
https://pubmed.ncbi.nlm.nih.gov/34459725/
1 patient, J&J Dose #1
Autopsy #14: Fatal cerebral haemorrhage after COVID-19 vaccine (Bjørnstad-Tuveng et al)14
https://pubmed.ncbi.nlm.nih.gov/33928772/
1 patient, AstraZeneca Dose unspecified
Autopsy #15: Lymphohistocytic myocarditis after Ad26.COV2.S viral vector COVID-19 vaccination (Ujueta et al)15
https://pubmed.ncbi.nlm.nih.gov/34514078/
1 patient, J&J Dose #1
Autopsy #16: Fatal intracranial hemorrhage in patient with thrombocytopenia and positive HIT antibodies after vaccination with ChAdOx1 nCoV-19 - VITT without thrombosis? (Medić et al)16
https://pubmed.ncbi.nlm.nih.gov/35640515/
1 patient, AstraZeneca Dose #1
Autopsy #17: Fatal Multisystem Inflammatory Syndrome in Adult after SARS-CoV-2 Natural Infection and COVID-19 Vaccination (Grome et al)17
https://pubmed.ncbi.nlm.nih.gov/34586059/
1 patient, Pfizer Dose #2
Category #2: Case Series
The following are case reports documenting at least 3 cases of a fatal vaccine-associated adverse event:
CS #1: Towards the emergence of a new form of the neurodegenerative Creutzfeldt-Jakob disease: Twenty six cases of CJD declared a few days after a COVID-19 vaccine Jab (Perez et al)18
https://www.researchgate.net/publication/363484655
26 patients (20 deaths), Pfizer Dose #1 x4 / Dose #2 x14 / Dose #3 x2; AstraZeneca Dose #2 x2, unknown Dose x1; Moderna Dose #2 x2 / Dose #1 x1
CS #2: Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination (Greinacher et al)19
https://pubmed.ncbi.nlm.nih.gov/33835769/
11 patients, 6/11 died, see chart in footnote for vaccination details
CS #3: Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination (Schultz et al)20
https://pubmed.ncbi.nlm.nih.gov/33835768/
5 patients, all AstraZeneca Dose #1
CS #4: Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination (Scully et al)21
https://pubmed.ncbi.nlm.nih.gov/33861525/
7/23 patients died, all AstraZeneca Dose #1
CS #5: Vaccine Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis With High Fatality Rate: A Case Series (Wiedmann et al)22
https://pubmed.ncbi.nlm.nih.gov/34393988/
4/5 patients died, all AstraZeneca Dose #1
CS #6: Case Series: Acute Hemorrhagic Encephalomyelitis After SARS-CoV-2 Vaccination (Ancau et al)23
https://pubmed.ncbi.nlm.nih.gov/35185757/
2/3 patients died, all AstraZeneca Dose #1
Category #3: Transplanting Organs from VITT Victims
The following are case reports documenting cases where organ transplants from patients killed by VITT caused by a covid vaccine were either performed or at least considered Patient/s listed refers to donor/s killed by vaccine:
T-VITT #1: Organ Donation From a Brain Dead Donor With Vaccine-induced Immune Thrombotic Thrombocytopenia After Ad26.COV2.S: The Risk of Organ Microthrombi (Uzun et al)24
https://pubmed.ncbi.nlm.nih.gov/34974451/
1 patient, J&J Dose #1
T-VITT #2: Fatal cerebral venous sinus thrombosis after COVID-19 vaccination (Jamme et al)25
https://pubmed.ncbi.nlm.nih.gov/33983464/
1 patient, AstraZeneca Dose #1
T-VITT #3: Vaccine-Induced Thrombotic Thrombocytopenia Due to Coronavirus Disease 2019 Vaccine From a Deceased Donor: A Case Report (Guditi et al)26
https://pubmed.ncbi.nlm.nih.gov/34916063/
1 patient, AstraZeneca Dose #1
T-VITT #4: Outcome After Organ Transplantation From Brain-dead Donors After a Cerebral Insult Following SARS-CoV-2 Vaccination Within the Eurotransplant Region27
https://pubmed.ncbi.nlm.nih.gov/34570086/
6 patients, AstraZeneca, Dose unspecified x5; J&J Dose #1 x1
T-VITT #5: Successful Liver Transplantation From a Deceased Donor With Vaccine-Induced Thrombotic Thrombocytopenia Causing Cerebral Venous Sinus and Hepatic Veins Thrombosis After ChAdOx1 nCov-19 Vaccination (Centonze et al)28
https://pubmed.ncbi.nlm.nih.gov/34172647/
1 patient, AstraZeneca Dose #1
T-VITT #6: Transplantation Outcome in Recipients Engrafted With Organs Recovered From the First French Deceased Donor With a SARS-COV-2 Vaccine-induced Thrombotic Thrombocytopenia (Jamme et al [#2])29
https://pubmed.ncbi.nlm.nih.gov/34115658/
1 patient, AstraZeneca Dose #1
T-VITT #7: New-Onset Antibodies to Platelet Factor 4 Following Liver Transplantation From a Donor With Vaccine-Induced Thrombotic Thrombocytopenia (Valsecchi et al)30
https://pubmed.ncbi.nlm.nih.gov/34416086/
1 patient, vaccine unknown but presumed to be one of the Big 4 because all authors are from Italy (paywall)
T-VITT #8: Organ transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia (Greenhall et al)31
https://pubmed.ncbi.nlm.nih.gov/34214257/
13 patients, AstraZeneca Dose #1 x13
T-VITT #9: Solid organ procurement and transplantation from deceased donors with vaccine-induced thrombosis and thrombocytopenia (Loupy et al)32
https://pubmed.ncbi.nlm.nih.gov/34233058/
Category #4: Otherwise Uncategorized Case Reports
The following are case reports documenting a death following a covid vaccine-associated adverse event:
CR #1: Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: a catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia (De Michele et al)33
https://pubmed.ncbi.nlm.nih.gov/34341358/
1 patient, AstraZeneca Dose #1
CR #2: Intracerebral Hemorrhage due to Thrombosis with Thrombocytopenia Syndrome after Vaccination against COVID-19: the First Fatal Case in Korea (Choi JK et al)34
https://pubmed.ncbi.nlm.nih.gov/34402235/
1 patient, AstraZeneca Dose #1
CR #3: Fatal vaccine-induced immune thrombotic thrombocytopenia (VITT) post Ad26.COV2.S: first documented case outside US (Rodriguez et al)35
https://pubmed.ncbi.nlm.nih.gov/34626338/
1 patient, J&J Dose #1
CR #4: Brain death in a vaccinated patient with COVID-19 infection (Watchmaker & Belani)36
https://pubmed.ncbi.nlm.nih.gov/34656887/
1 patient, Pfizer Dose #2
CR #5: Fatal myositis, rhabdomyolysis and compartment syndrome after ChAdOx1 nCoV-19 vaccination (Huang ST et al)37
https://pubmed.ncbi.nlm.nih.gov/35504826/
1 patient, AstraZeneca Dose #2
CR #6: Fatal intracerebral haemorrhage associated with thrombosis with thrombocytopenia syndrome after ChAdOx1-S vaccine (Gómez-Roldós et al)38
https://pubmed.ncbi.nlm.nih.gov/36169326/
1 patient, AstraZeneca Dose #1
CR #7: Cerebral Venous Sinus Thrombosis With Severe Thrombocytopenia: A Fatal Adverse Event After Johnson & Johnson COVID-19 Vaccination (Ali M et al)39
https://cp.neurology.org/content/11/6/e971
1 patient, J&J Dose #1
CR #8: Fatal exacerbation of ChadOx1-nCoV-19-induced thrombotic thrombocytopenia syndrome after initial successful therapy with intravenous immunoglobulins - a rational for monitoring immunoglobulin G levels (Douxfils et al)40
https://pubmed.ncbi.nlm.nih.gov/34847660/
1 patient, AstraZeneca Dose #1
CR #9: Vaccine-Induced Immune Thrombotic Thrombocytopenia with Disseminated Intravascular Coagulation and Death following the ChAdOx1 nCoV-19 Vaccine (Aladdin et al)41
https://pubmed.ncbi.nlm.nih.gov/34171649/
1 patient, AstraZeneca Dose #1
CR #10: First and fatal case of autoimmune acquired factor XIII/13 deficiency after COVID-19/SARS-CoV-2 vaccination (Shimoyama et al)42
https://pubmed.ncbi.nlm.nih.gov/34856014/
1 patient, Pfizer Dose #2
CR #11: Fatal Outcome of COVID-19 Relapse in a Fully Vaccinated Patient with Non-Hodgkin Lymphoma Receiving Maintenance Therapy with the Anti-CD20 Monoclonal Antibody Obinutuzumab: A Case Report (Calò et al)43
https://pubmed.ncbi.nlm.nih.gov/35891185/
1 patient, Pfizer Dose #3
CR #12: Acute transverse myelitis after BNT162b2 vaccination against COVID-19: Report of a fatal case and review of the literature (Nakano et al)44
https://pubmed.ncbi.nlm.nih.gov/34953348/
1 patient, Pfizer Dose #2
CR #13: An Interesting Case of Fatal Myasthenic Crisis Probably Induced by the COVID-19 Vaccine (Sonigra et al)45
https://pubmed.ncbi.nlm.nih.gov/35449619/
1 patient, AstraZeneca Dose #1
CR #14: A Case of Heart Transplantation for Fulminant Myocarditis After ChAdOx1 nCoV-19 Vaccination (Kim SH et al)
https://pubmed.ncbi.nlm.nih.gov/35380028/46
1 patient, AstraZeneca Dose #2
CR #15: Ischaemic stroke as a presenting feature of ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia (Al-Mayhani et al)47
https://pubmed.ncbi.nlm.nih.gov/34035134/
1 patient, AstraZeneca Dose unspecified
CR #16: [Intracerebral haemorrhage twelve days after vaccination with ChAdOx1 nCoV-19] (Wolthers et al)48
https://pubmed.ncbi.nlm.nih.gov/34477089/
1 patient, AstraZeneca Dose unspecified
CR #19: A Single-Health System Case Series of New-Onset CNS Inflammatory Disorders Temporally Associated With mRNA-Based SARS-CoV-2 Vaccines (Ballout et al)49
https://pubmed.ncbi.nlm.nih.gov/35280277/
1 patient, Moderna Dose #1
Case Reports. Patient 1 was a 50-year-old man (body weight 90 kg) with abdominal pain that developed 10 days after vaccination with ChAdOx1 nCoV-19. He had neither a history for thrombosis risk factors nor had he any intake of drugs increasing this risk. At the emergency room he presented with severe thrombocytopenia, low plasma fibrinogen and very high D-dimer (Table 1). The results of other blood tests were normal except for moderately elevated white blood cells and inflammatory serum markers. Computed tomography (CT) showed portal vein thrombosis with smaller thrombi in the splenic and upper mesenteric veins. During the next 4 days after admission platelets and fibrinogen remained low and D-dimer very high with no substantial changes. An initial dose of the low molecular weight heparin nadroparin was given subcutaneously at a dosage of 5,700 IU followed by a second dose after 8 hours. Clinical conditions deteriorated and a new CT scan showed massive intracerebral hemorrhage. Treated with multiple transfusions of platelet concentrates that failed to control bleeding the patient died 4 days after the onset of symptoms and 16 days after vaccination. A serum sample obtained before nadroparin showed the presence of anti PF4/polyanion complex IgG antibodies by enzyme-linked immunosorbent assay (ELISA) (Lifecodes PF4 IgG assay, Immucor, USA).
Patient 2, a 37-year old previously healthy woman (61 kg) with a negative history for significant disease and drug intake developed 10 days after the administration of the same vaccine first low back pain and then a strong headache. She became progressively drowsy and ultimately unconscious, and was, therefore, admitted to the emergency room of her local hospital. With laboratory tests similar of those of patient 1 (Table 1), a CT scan showed an occlusive thrombus in the superior sagittal venous sinus and a very large hemorrhage in the frontal cerebral lobe. Transported comatose by helicopter to a larger hub hospital she underwent craniotomy in order to control intracranial hypertension and remove the frontal lobe hemorrhage. She survived the operation but remained comatose and died 10 days after the first hospital admission and 23 days after vaccination. Anti- PF4/polyanion complex antibody reactivity was detected by ELISA and confirmed in a stored serum sample (Table 1).
A 27-year-old man was transferred to the emergency room in cardiopulmonary arrest. The patient had just received the first dose of the mRNA-1273 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (Moderna, Cambridge, MA, USA) 8 days earlier and had no symptoms such as chest pain suspected of myocarditis or general fatigue suspected of low cardiac output after the vaccination until the emergency room visit. He had a high exercise habit; his teammates called for an ambulance when he was found sitting unconscious during practice. Upon arrival at the emergency room, he presented asystole. Despite cardiopulmonary resuscitation, fatal ventricular arrhythmias (Fig. 1A) repeated, and he eventually received venoarterial extracorporeal membrane oxygenation (VA-ECMO) and Impella CP (Abiomed, Danvers, MA, USA), after 2 h of his visit. A chest radiograph obtained in an emergency room showed an enlarged heart and pulmonary congestion (Fig. 1E). The patient had undergone orthopedic surgery 3 months before the recent hospitalization. An electrocardiogram (ECG) abnormality and mild cardiac shadow enlargement in chest radiograph had been noted preoperatively (Fig. 1B, D); however, since he was asymptomatic, no further examination was performed. Additionally, he had no family history of cardiovascular disease or sudden death.
A 61-year-old woman without significant medical history developed fever 3 days after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and went into shock the next day. She was negative for SARS-CoV-2 mRNA in real-time polymerase chain reaction (PCR). Finally, she died 10 days after vaccination. At autopsy, the heart showed moderate dilatation of both ventricles, and the myocardium showed an uneven color change and decreased elasticity. Histologically, severe myocarditis with extensive myocytolysis was observed. The myocarditis showed severe inflammatory cell infiltration with T-lymphocyte and macrophage predominance, and in addition to the inflammatory cells described above, vast nuclear dust accompanying neutrophilic infiltration was observed. In the bone marrow and lymph nodes, hemophagocytosis was observed. In postmortem examination, nucleic acids of any cardiotropic viruses including SARS-CoV-2 were not detected using multivirus real-time PCR system. We discussed the relationship between the possible immune reaction after vaccination and the myocarditis observed in this case from immunopathological viewpoints. This mRNA vaccine is the first applied nucleic acid vaccine for humans, and its mechanism of efficacy and immune acquisition remain unclear. We hope the accumulation of more detailed analyses of the similar cases to reveal the mechanism of this kind of adverse reaction.
Case presentation: A 72-year-old woman with a history of diffuse large B-cell lymphoma in the stomach and hyperthyroidism received the first dose of the BNT162b2 mRNA vaccine and died 2 days later. The autopsy revealed multiple microthrombi in the heart, brain, liver, kidneys, and adrenal glands. The thrombi were CD61 and CD42b positive and were located in the blood vessels primarily in the pericardial aspect of the myocardium and subcapsular region of the adrenal glands; their diameters were approximately 5-40 μm. Macroscopically, a characteristic myocardial haemorrhage was observed, and the histopathology of the characteristic thrombus distribution, which differed from that of haemolytic uraemic syndrome and disseminated intravascular coagulation, suggested that the underlying pathophysiology may have been similar to that of thrombotic microangiopathy (TMA).
Conclusion: This is the first report on a post-mortem case of multiple thromboses after the BNT162b2 mRNA vaccine. The component thrombus and characteristic distribution of the thrombi were similar to those of TMA, which differs completely from haemolytic uraemic syndrome or disseminated intravascular coagulation, after vaccination. Although rare, it is important to consider that fatal adverse reactions may occur after vaccination and that it is vital to conduct careful follow-up.
The current report represents a case of a 77-year-old man with Parkinson’s disease who died three weeks after receiving his third COVID-19 vaccination in January 2022. The patient was first vaccinated in May 2021 with the ChAdOx1 nCov- 19 vector vaccine, followed by two more doses with the BNT162b2 mRNA vaccine in July and December 2021. The family of the deceased requested an autopsy due to the ambivalent clinical features noted before death. The underlying illness (Parkinson’s disease) was confirmed by autopsy. However, no sign of a florid COVID-19 was discovered. Meanwhile, the immunohistochemical staining of the brain and heart revealed previously undiagnosed conditions. The brain, in distinctive, revealed multifocal necrotizing encephalitis with massive inflammatory lymphocyte infiltrates. In addition, the heart showed signs of serious myocarditis. Finally, immunohistochemical staining revealed that the SARS-CoV-2 spike protein was evident in the tissues investigated. Based on these immunohistochemical findings, it appears that the inflammatory changes in the patient's brain tissues are most likely the result of immunological processes. Concurrently, the absence of SARS-CoV-2 nucleocapsid-protein was evidenced, indicating that the detected spike-protein is unrelated to a SARS-CoV-2 infection. If such an infection was the cause of the spike protein, the SARS-CoV-2 nucleocapsid protein would also be detectable. As a consequence, the confirmed presence of the spike protein had to be attributed to the previous vaccination with the BNT162b2 mRNA vaccine that the deceased patient had received.
We present autopsy findings of a 22-year-old man who developed chest pain 5 days after the first dose of the BNT162b2 mRNA vaccine and died 7 hours later. Histological examination of the heart revealed isolated atrial myocarditis, with neutrophil and histiocyte predominance. Immunohistochemical C4d staining revealed scattered single-cell necrosis of myocytes which was not accompanied by inflammatory infiltrates. Extensive contraction band necrosis was observed in the atria and ventricles. There was no evidence of microthrombosis or infection in the heart and other organs. The primary cause of death was determined to be myocarditis, causally-associated with the BNT162b2 vaccine.
Results.—
The microscopic examination revealed features resembling a catecholamine-induced injury, not typical myocarditis pathology.
Conclusions.—
The myocardial injury seen in these postvaccine hearts is different from typical myocarditis and has an appearance most closely resembling a catecholamine-mediated stress (toxic) cardiomyopathy. Understanding that these instances are different from typical myocarditis and that cytokine storm has a known feedback loop with catecholamines may help guide screening and therapy.
Myocarditis in adolescents (particularly teenage boys) has been reported following the second dose of the Pfizer-BioNTech COVID-19 vaccine. Since cardiac biopsies are rarely performed in these instances with clinically stable patients, the myocardial pathology has not been clearly elucidated. Myocarditis is rarely diagnosed at autopsy in deaths due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The incidence of myocarditis, although low, has been shown to increase after the receipt of the BNT162b2 vaccine, particularly after the second dose among young male recipients. In addition, the first week after the second vaccine dose was found to be the main risk window. The clinical presentation of myocarditis after vaccination was usually mild.
We report the autopsy results, including microscopic myocardial findings, of 2 teenage boys who died within the first week after receiving the second Pfizer-BioNTech COVID-19 dose. The microscopic findings are not the alterations seen with typical myocarditis. This suggest a role for cytokine storm, which may occur with an excessive inflammatory response, as there also is a feedback loop between catecholamines and cytokines.
This case report describes a fatal case of a young woman with superior sagittal, transverse and sigmoid sinus thrombosis after administration of the ChAdOx1 nCov-19 vaccination. Eleven days post-vaccination she was found unconscious and transferred to the Emergency Department. Blood parameters showed low platelets, and a CT scan showed an extensive left intracranial hemorrhage and the presence of an occlusive thrombus of the superior sagittal sinus. She under-went a craniectomy, but after the intervention, she remained in a comatose state. After a few days, her clinical conditions worsened, and she died. A complete autopsy was performed which showed a thrombosis of the cerebral venous district, of the upper and lower limbs. A blood sample was also performed to carry out a gene study about the predisposition to thrombosis. The organ samples were studied through light microscope both in hematoxylin-eosin and immunohistochemical examination, and showed a strong inflammatory response in all samples and at the site of thrombosis. Our study aims to provide a proper autopsy technique to study the entire cerebral venous system through a multidisciplinary approach (anatomical dissection and neurosurgery) in post-vaccine venous thrombosis.
The clinical and pathological observations from a case of fatal fulminant necrotising myocarditis in a 57-year-old woman, following the first dose of the Pfizer-BioNTech vaccine, are described. Other causes have been discounted with reasonable certainty.
Histological examination of the heart sections showed fulminant necrotizing eosinophilic myocarditis (Fig. 1, bottom left and bottom right). There were multifocal aggregates of lymphoid cells, histiocytes and abundant eosinophils with focal myocyte necrosis in the free walls of both ventricles, inter-ventricular septum and around the conduction system (sino-atrial and atrio-ventricular nodes). No parasitic organisms or giant cells were identified. The eosinophilic infiltrate would make autoimmune myocarditis less likely. There was no evidence of eosinophils in other organs or eosinophilic vasculitis. Histological examination of the left pleural space mass showed a thymoma, WHO subtype AB.
CASE REPORT: A 58-year-old man, after 13 days from the first administration of ChAdOx1 nCoV-19 vaccine (AstraZeneca), presented with abdominal pain, diarrhea and vomitus. Laboratory tests revealed a severe thrombocytopenia, low fibrinogen serum levels and marked increase of D-dimer serum levels. The patient quickly developed a multiple organ failure, till death, three days after the hospital admission. RESULTS: At histology, in the lungs, interalveolar septa appeared thickened with microthrombi in the capillaries and veins. Interalveolar septa appeared thickened and showed vascular proliferation. Thrombi were detected in the capillaries of glomerular tufts. In the hearth, thrombi were observed in veins and capillaries. In the liver, voluminous fibrin thrombi were diffusely observed in the branches of the portal vein. Microthrombi were also found in the vasa vasorum of the wall of abdominal aorta. In the brain, microthrombi were observed in the capillaries of the choroid plexuses. Diffuse hemorrhagic necrosis was observed in the intestinal wall with marked congestion of the venous vessels.
CONCLUSIONS: In our patient, the majority of data necessary for a VITT final diagnosis were present: thrombocytopenia and thrombosis in pulmonary, portal, hepatic, renal and mesenteric veins, associated with a marked increase of D-dimer serum levels. The finding of cerebral thrombosis in choroid plexuses, is a new finding in VITT. These features are suggestive for a very aggressive form of VITT.
We report a case of a 62-year-old man who developed cerebral venous sinus thrombosis with subarachnoid hemorrhage and concomitant thrombocytopenia, which occurred 13 days after ChAdOx1 nCov-19 injection. The patient died in the intensive care unit after heparin infusion and platelet transfusion.
The results of the autopsy confirmed death by superior sagittal sinus thrombosis with subarachnoid hemorrhage and a rupture of the thrombosed cortical vein. The autopsy also confirmed multivisceral thrombosis in small, medium, and large vessels in many organs, such as the heart (Figure 1A), the liver (Figure 1B), the kidney, and the spleen, accompanied by typical lesions of ischemic myocardial and hepatic necrosis. Macroscopically, the heart and liver appeared heterogeneous. Microscopically, in the liver, we observed the presence of steatosis (30%), central lobular necrosis, and vascular and endothelial thrombi in medium‐sized vessels. In the heart, there was gross morphologic evidence of early myocardial infarction, with an organizing thrombus in a branch of the left circumflex coronary. A standard immunohistochemical method detected a heterogeneous desmin depletion. The blood vessels had clumped red blood cells in the lumen, forming fibrino‐cruoric microthrombi.
We report a case of a Japanese man with severe rhabdomyolysis and multiple thrombosis of arterioles after the first dose of mRNA-1273 vaccine. He developed rapidly progressive rhabdomyolysis and infarctions of multiple organs. Antiplatelet factor 4 antibody test was negative. Despite the intensive supportive care, including aggressive fluid administration, hemodialysis, administration of anticoagulants, high-dose steroid, and eculizumab, the patient ultimately died of multiple organ failure.
Autopsy revealed acute to subacute liver infarction with scattered white changes surrounded by hemorrhagic areas (Figure 1D). Both iliopsoas muscles and the right quadriceps femoris were extensively necrotic with massive hemorrhage. Histological examination revealed multiple small arterial thrombosis, degeneration, and necrosis of the myocytes and hepatocytes (Figure 1E, E,1F).1F). The extensive hemorrhagic necrosis of the mucosa that involved the whole intestinal tract was thought to be the consequence of thrombosis of involved arterioles (Figure 1G, arrow head). Microscopic findings were consistent with the features of a thrombotic microangiopathy (TMA). Myoglobin deposition was observed in the renal tubules (Figure 1H). Immunofluorescence revealed the deposition of C3 in the glomeruli (Figure 1I). The anti-PF4 antibody measured by ELISA was negative. Finally, we diagnosed the patient with vaccine-induced TMA with rhabdomyolysis as an initial symptom.
A 38-year-old man reporting vomiting and dizziness sought treatment at an emergency department. Smoldering multiple myeloma had been diagnosed 1.5 years before, but no laboratory abnormalities had been found in his most recent hospital visit 5 months earlier. He had received the Ad26.COV2.S vaccine 2 days before the emergency department visit and experienced fever, chills, and myalgia 12–24 hours postvaccination, then nausea, recurrent vomiting, and general weakness 24–48 hours postvaccination. At admission, he was afebrile, his heart rate was 130 beats/min, and his blood pressure was 100/90 mm Hg, with no noticeable edema. We administered isotonic saline and initiated diagnostic evaluations: laboratory tests, imaging, and COVID-19 reverse transcription PCR. Test results (Table) showed marked hemoconcentration and hypoalbuminemia. Chest and abdominal computed tomography results were unremarkable. Six hours after admission, the patient was hypotensive (blood pressure 60/40 mm Hg), had a heart rate of 132 beats/min, and reported dyspnea. We obtained blood cultures and treated the patient with broad-spectrum antimicrobials, intravenous fluids, and inotropes. Despite these measures, the patient’s hypotensive shock worsened, and he died 10 hours after admission.
Although at admission the patient showed neither peripheral edema nor severe hypoalbuminemia, we suspected SCLS for several reasons. First, we could not entirely rule out infection, but results of blood cultures and COVID-19 testing were negative. Second, autopsy results showed no evidence of acute infection or cardiovascular disease in the internal organs. We identified pulmonary edema, pleural effusion, and pericardial effusion. Although pulmonary edema is atypical in acute SCLS attacks (leak phase), prolonged cardiopulmonary resuscitation and fluid administration might have affected the autopsy findings. Histopathologic findings in both kidneys suggested autolysis or acute tubular necrosis, which helped exclude other possible etiologies of refractory hypotensive shock.
We believe a life-threatening flare developed after COVID-19 vaccination in the patient in our study who had a history suggestive of SCLS. Clinical findings were compatible with a previous report in which life-threatening disease occurred 1–2 days after vaccination (3,6); we could identify no SCLS triggers other than receiving the COVID-19 vaccine.
Case presentation: The patient was a previously healthy woman in her thirties with headaches that developed one week after vaccination with ChAdOx1 nCoV-19. Three days later, her condition deteriorated rapidly, and she presented to the emergency department with slurred speech, uncoordinated movements and reduced consciousness. Symptoms progressed to left-sided hemiparesis and her level of consciousness deteriorated. Computed tomography (CT) of the head showed a large right-sided haemorrhage and incipient herniation. She was found to have severe thrombocytopenia 37 x 109/l, (ref 145 - 390 x 109/l). In spite of efforts to reduce intracranial pressure, the patient died the following day. Post mortem examination revealed antibodies to PF4, and fresh small thrombi were found in the transverse sinus, frontal lobe and pulmonary artery.
A 62-year-old Caucasian female visiting from Uruguay presented to the emergency department complaining of progressive body aches, weakness and worsening fatigue approximately 4 days after administration of the Ad26.COV2.S (Janssen Johnson & Johnson®) viral vector COVID-19 vaccine.
Bedside echocardiogram revealed a severe biventricular cardiomyopathy with left ventricular ejection fraction of 29%, and a small pericardial effusion with no evidence of increased intrapericardial pressure (supplementary Fig. 1A & B, Video Supplementary). Urgent cardiac angiography was completed which demonstrated a cardiac output 5.1 L/min, cardiac index 3.26 L/min/m2, left ventricular end-diastolic pressure 37 mmHg, and no obstructive disease on maximum dose of Norepinephrine. Right heart catheterization demonstrated pulmonary artery pressure of 36/30 mm Hg, with a mean of 33 mm Hg. The patient was transferred to the intensive care unit with increasing vasopressor support with maximal dosing of Vasopressin, Phenylephrine, and Epinephrine. Intravenous (IV) Methylprednisolone 60 mg bolus was administered every 8 h, and intravenous immunoglobulin was ordered but not administered [7]. A cardiac arrest code was called 18 h after presentation, and the patient expired after several rounds of advanced cardiovascular life support. Consent was obtained for an autopsy.
The autopsy was performed 9-hours postmortem. No remarkable changes were observed on the body surface. Autopsy revealed about 15 cc of straw-colored fluid in the pericardial sac. The heart weighed 250 g with unremarkable gross changes. No apparent coronary artery disease was appreciated. No focal lesions suggestive of acute or chronic hypoxic injury were identified. Microscopic view of the myocardial biopsy showing scattered positive CD3 immunostaining supporting T cell infiltration (Fig. 1b). Multiple immunohistochemistry staining like CD163 supports the diagnosis of lymphohistiocytic myocarditis with sparse eosinophils (Supplementary Fig. 2).
Both lungs were congested and heavy (right 630 g and left 650 g). There were 850 and 320 cc of serosanguineous fluid on right and left pleural cavities, respectively. Grossly, multiple nodules were identified in all lobes of the lungs, ranging from 0.5 to 1.5 cm in size. The cut surfaces showed a variety of consistencies and colors from a firm red pink to a semisolid white with grumous material. Sampling of tissue from all nodules showed multiple sites of metastasis of melanoma in both lungs. Examination of blood vessels revealed numerous sites of thrombotic microangiopathy.
This case suggests a potential relationship between the viral vector COVID-19 vaccine and the patient’s lymphohistiocytic myocarditis resulting in severe biventricular cardiomyopathy and death.
We present the case of a previously healthy young male patient that developed thrombocytopenia and prothrombotic state following vaccination with ChAdOx1 and died due to massive intracranial hemorrhage.
31-year-old, otherwise healthy man was admitted to the regional hospital for sudden sharp pain in both thoracolumbar regions that occurred during the night's rest after strenuous physical activity.
The patient received the first dose of anti-COVID -19 ChAdOx1 nCOV-19 vaccine 10 days earlier and developed fever and flu-like symptoms for 24 h. On the fourth day after vaccination, he developed diarrhea, and on seventh exacerbation of chronic sinusitis, and was treated with azithromycin.
We found persistent thrombocytopenia (Plts 66 × 10 e9/L), elevated fibrinogen (5.2 g/L) and d-dimers (17.62 mg/L), with normal PT and aPTT.
The next day, patient suddenly became confused and disorientated, and MSCT confirmed intracerebral hematoma in the temporal and occipital lobes (55 × 30 mm in diameter) with suspected thrombosis of sagittal sinus. Despite treatment with rFVIIa and platelet transfusions, intracerebral hematoma progressed, and the patient was transferred to OR for urgent removal of the hematoma. However, a couple of hours later patient become unstable with clinical signs of further progression of intracranial bleeding and died in cardiac arrest. Autopsy revealed diffuse cerebral edema, acute hemorrhage in the left frontotemporoparietal region and cerebellum, and diffuse subarachnoid hemorrhage. There was no evidence of thrombosis in the cerebral venous sinuses, renal or mesenteric veins. Microscopic analysis revealed no evidence of microangiopathy in the brain or other parenchymal organ.
Immunohematological testing to support the diagnosis of VITT was performed 47 days post mortem. Enzyme-linked immunosorbent assays (ELISA) for heparin induced thrombocytopenia (HIT) (PF4 IgG Kit; GTI Diagnostics, Waukesha, Wisconsin, USA) showed positive result: optical density (OD, 450 nM): 1618; threshold for positive test, >0.4). A functional assay was then preformed to detect platelet-activating antibodies directed against PF4/heparin using flow cytometer (Fig. 1a, b, c).
In our patient, no signs of thrombosis were found even on autopsy. To our knowledge, this is the first case of VITT described in the literature that has no proven thrombosis.
We describe a fatal case of multisystem inflammatory syndrome in an adult with onset 22 days after a second dose of mRNA coronavirus disease vaccine. Serologic and clinical findings indicated severe acute respiratory syndrome coronavirus 2 infection occurred before vaccination.
Twenty-two days after receiving the second dose of the COVID-19 vaccine, he had onset of new fever, malaise, headache, and odynophagia. He was examined by an outpatient medical provider. Diagnostic testing was notable for a negative COVID-19 test by reverse transcription PCR (RT-PCR), negative rapid influenza antigen, and negative rapid antigen detection for group A Streptococcus. Four days later, the patient visited an emergency department because of worsening symptoms. Assessment of vital signs revealed a temperature of 37.2°C, heart rate 113 beats/min, and blood pressure of 117/66 mmHg. Physical examination identified right-sided cervical lymphadenopathy, marked bilateral conjunctival erythema, and a faint papular rash on the pelvis and left flank. Laboratory testing revealed a peripheral-blood leukocyte count of 11,000 cells/μL, 93.5% segmented neutrophils, and thrombocytopenia with a platelet count of 110,000/μL (Table).
Early morning on hospital day 3, the patient had an acute change in mental status, including confusion and global aphasia. An emergent computed tomography scan of the head was negative for cerebrovascular accident and showed normal brain parenchyma and no evidence of acute infarction, mass, or hemorrhage. On completion of the scan, the patient was found nonresponsive and without a pulse. He underwent multiple rounds of advanced cardiac life support, resulting in return of spontaneous circulation. A chest radiograph showed an enlarged cardiac silhouette, and an echocardiogram showed severe biventricular dysfunction, severe global hypokinesis of the left ventricle, and left ventricular ejection fraction of 20%. The patient received a second dose of IVIg and intravenous steroids and extracorporeal membrane oxygenation support was initiated. On hospital day 4, severe multisystem organ failure continued to progress. The patient died on hospital day 4.
To summarize, of the 26 cases analyzed, the first symptoms of CJD appeared on average 11.38 days after the injection of the COVID-19 "vaccine". Of these 26 cases, 20 had died at the time of writing this article while 6 were still alive. The 20 deaths occurred only 4.76 months after the injection. Among them, 8 of them lead to a sudden death (2.5 months). All this confirms the radically different nature of this new form of CJD, whereas the classic form requires several decades.
Results: Of the 11 original patients, 9 were women, with a median age of 36 years (range, 22 to 49). Beginning 5 to 16 days after vaccination, the patients presented with one or more thrombotic events, with the exception of 1 patient, who presented with fatal intracranial hemorrhage. Of the patients with one or more thrombotic events, 9 had cerebral venous thrombosis, 3 had splanchnic-vein thrombosis, 3 had pulmonary embolism, and 4 had other thromboses; of these patients, 6 died. Five patients had disseminated intravascular coagulation. None of the patients had received heparin before symptom onset. All 28 patients who tested positive for antibodies against PF4–heparin tested positive on the platelet-activation assay in the presence of PF4 independent of heparin. Platelet activation was inhibited by high levels of heparin, Fc receptor–blocking monoclonal antibody, and immune globulin (10 mg per milliliter). Additional studies with PF4 or PF4–heparin affinity purified antibodies in 2 patients confirmed PF4-dependent platelet activation.
Conclusions: Vaccination with ChAdOx1 nCov-19 can result in the rare development of immune thrombotic thrombocytopenia mediated by platelet-activating antibodies against PF4, which clinically mimics autoimmune heparin-induced thrombocytopenia. (Funded by the German Research Foundation.)
We report findings in five patients who presented with venous thrombosis and thrombocytopenia 7 to 10 days after receiving the first dose of the ChAdOx1 nCoV-19 adenoviral vector vaccine against coronavirus disease 2019 (Covid-19). The patients were health care workers who were 32 to 54 years of age. All the patients had high levels of antibodies to platelet factor 4–polyanion complexes; however, they had had no previous exposure to heparin. Because the five cases occurred in a population of more than 130,000 vaccinated persons, we propose that they represent a rare vaccine-related variant of spontaneous heparin-induced thrombocytopenia that we refer to as vaccine-induced immune thrombotic thrombocytopenia.
We report findings in 23 patients who presented with thrombosis and thrombocytopenia 6 to 24 days after receiving the first dose of the ChAdOx1 nCoV-19 vaccine (AstraZeneca). On the basis of their clinical and laboratory features, we identify a novel underlying mechanism and address the therapeutic implications.
During a 2-week period, we have encountered five cases presenting with the combination of cerebral venous thrombosis (CVT), intracerebral hemorrhage and thrombocytopenia. A clinical hallmark was the rapid and severe progression of disease in spite of maximum treatment efforts, resulting in fatal outcome in for 4 out of 5 patients. All cases had received ChAdOx1 nCov-19 vaccine 1–2 weeks earlier and developed a characteristic syndrome thereafter. The rapid progressive clinical course and high fatality rate of CVT in combination with thrombocytopenia in such a cluster and in otherwise healthy adults is a recent phenomenon. Cerebral autopsy findings were those of venous hemorrhagic infarctions and thrombi in dural venous sinuses, including thrombus material apparently rich in thrombocytes, leukocytes and fibrin. Vessel walls were free of inflammation. Extra-cerebral manifestations included leech-like thrombi in large veins, fibrin clots in small venules and scattered hemorrhages on skin and membranes. CVT with thrombocytopenia after adenovirus vectored COVID-19 vaccination is a new clinical syndrome that needs to be recognized by clinicians, is challenging to treat and seems associated with a high mortality rate.
We present three cases fulfilling diagnostic criteria of hemorrhagic variants of acute disseminated encephalomyelitis (acute hemorrhagic encephalomyelitis, AHEM) occurring within 9 days after the first shot of ChAdOx1 nCoV-19. AHEM was diagnosed using magnetic resonance imaging, cerebrospinal fluid analysis and brain biopsy in one case. The close temporal association with the vaccination, the immune-related nature of the disease as well as the lack of other canonical precipitating factors suggested that AHEM was a vaccine-related adverse effect. We believe that AHEM might reflect a novel COVID-19 vaccine-related adverse event for which physicians should be vigilant and sensitized.
Case 1: A 61-year-old male with a history of hypothyroidism and polymyalgia rheumatica developed fever, headache and apathy 2 days after the first shot of the ChAdOx1 nCoV-19 vaccine. Two days later, he was discovered by his wife unconscious in his bed foaming around the mouth. Upon arrival of the emergency doctor, he exhibited a generalized seizure, was subsequently comatose and underwent endotracheal intubation. A head CT scan including CT-angiography revealed diffuse hypodense areas in the right subcortical frontotemporal and the right thalamic region. There were no signs of vessel occlusions, especially no sinus vein thrombosis. Magnetic resonance imaging (MRI) revealed bilateral confluent cortical and subcortical FLAIR hyperintense lesions with hemorrhagic involvement of the basal ganglia (Figure 1B).
We assumed an episode of AHEM and administered high dose steroid treatment (1 g methylprednisolone intravenously per day) over 5 days, followed by seven plasma exchange sessions (Figure 1A) with concomitant methylprednisolone administration (250 mg per day via nasogastric tube on days of plasma exchange, 100 mg per day via nasogastric tube on days between plasma exchanges, followed by tapering beginning with 100 mg orally per day and subtraction of 20 mg every 2 days), upon which there was slight improvement of alertness and reduction in size of the brain lesions in follow-up MRI already after 5 days. On clinical follow-up after 14 weeks of rehabilitation, the patient presented with a vegetative state.
Case 3: A 55-year-old woman developed progressive nausea, dizziness and meningism 9 days after the first shot of the ChAdOx1 nCoV-19 vaccine. Symptoms worsened rapidly to severe spastic tetraparesis and coma. Brain MRI revealed multiple FLAIR-hyperintense and hemorrhagic lesions in the right parietal and temporal lobes, bilaterally in fronto-temporal distribution as well as in the right occipital lobe and left fronto-basal region (Figure 1D).
The patient was put on a high dose steroid treatment (1 g methylprednisolone intravenously per day over 5 days) with subsequent tapering over 10 days (beginning with 100 mg via nasogastric tube per day and subtracting 20 mg every 2 days), which led to significant improvement of vigilance and motor function (Figure 1A). Two weeks after steroid therapy, her state worsened again due to new brainstem and occipital FLAIR-hyperintense and hemorrhagic lesions. A repeat high dose steroid treatment remained without positive effects and the patient died due to progressive intracerebral hemorrhage of the brain stem. An autopsy was declined by members of the family of the patient.
A 50-y-old female developed VITT with severe thrombocytopenia and thrombosis of the cerebral arteries and venous sinuses 12 d after the Ad26.COV2.S (Johnson & Johnson) vaccine. A computed tomography (CT) scan showed extensive ischemia of both cerebral hemispheres and incipient entrapment of the brain stem. CT angiography revealed occlusion of the middle cerebral artery on both sides, as well as sinus vein thrombosis of the superior sagittal sinus and transverse sinus on both sides. No intracranial hemorrhage was detected. Initial platelet count was 55 x 109/L, and d-dimer level was 31 µg/mL. Activated partial thromboplastin time (22 s) and fibrinogen (378 mg/dL) were within a normal range. Antiplatelet factor 4 (anti-PF4)/heparin antibodies were detected (3.48 optical density [OD] units; negative <0.3 OD). Modified heparin-induced platelet activation assay was positive. The patient received IVIG therapy and argatroban. Brain death was determined on day 3 of hospitalization. After multidisciplinary discussion, we decided to evaluate the patient for organ donation.
Organ donation from deceased donors with VITT is associated with some risks, such as the presence of retained thrombi in the transplanted organ and the transmission of VITT to the recipient.3,5 Although our patient had normal biochemical parameters and ultrasonography of the kidneys was normal, we detected an infarction and microthrombi during further diagnostics. Furthermore, an intraluminal blood clot was detected in the liver after organ procurement. A thorough radiologic assessment of the organs with multiphase CT angiography before organ retrieval could be useful to detect hypoperfusion or infarct in organs. The extent of microthrombi and organ function should be carefully assessed before deciding whether the organ is suitable for transplantation or not.
A 69-year-old woman with arterial hypertension treated daily by hydrochlorothiazide and angiotensin receptor antagonist received a first dose of Oxford–AstraZeneca vaccine.
Eleven days after the vaccination, the patient developed headache associated with behavioral symptoms. At day 13, her daughter found her unconscious. Physical examination revealed a coma Glasgow 4/15, right mydriasis, bilateral Babinski reflex without hemodynamic instability or respiratory failure. She was intubated and transferred in our intensive care unit.
Immediate CT scan followed by MRI highlighted a severe bilateral frontal hemorrhage with brain herniation complicating a cerebral venous thrombosis of the left internal jugular vein, sigmoid sinus and superior sagittal sinus (Fig. (Fig.1).1). Moreover, thoracic CT scan showed concomitant segmentary pulmonary embolism. Blood analysis at admission revealed an isolated thrombopenia measured at 18G/L with positive anti-PF4 antibodies.
Evolution was dramatically poor in the next few hours with brain death, leading to an organ donation procedure.
An 18-year-old woman presented to the hospital on June 9, 2021, with symptoms of headache and fever of 3 days duration, vomiting and swaying while walking of 1 day duration, and 1 episode of a seizure on the day of presentation. She had received the first dose of COVID-19 vaccine (AstraZeneca, University of Oxford, and Serum Institute of India) on May 30, 2021.
Her sensorium worsened, and she gradually developed hypotension. She was treated with vasopressors, noradrenaline and dopamine infusions at 5 mL/hour, single-donor platelet transfusions, packed red blood cells transfusions, heparin 5000 unit every 8 hours, injection levetiracetam 500 mg infusion twice daily, injection piperacillin tazobactam 500 mg 3 times daily, injection mannitol 100 mg infusion every 24 hours, folvite 10 mg tablet daily, injection vitamin B12 once daily, and supportive care. Tracheostomy was done after a week, and she was continued on mechanical ventilation. VITT was considered because of the patient's thrombocytopenia, increased D-dimer, and thrombotic complications with a history of the COVID-19 vaccine a week before presentation and because no other cause explained her condition. However, an antibody against PF4 was negative. On June 19, 2021, the patient's condition worsened; her pupils were bilaterally dilated at 6 mm and fixed, and brain stem reflexes were absent. Electroencephalography showed isoelectric activity consistent with electrocerebral silence, without any discernible activity while recording at a sensitivity of 2uV. An apnea test was performed to confirm the diagnosis of brain death; both the apnea tests done 6 hours apart were positive, confirming the diagnosis of brain death. Brainstem death declaration was done on June 19, 2021. The patient's parents were counseled regarding the organ donation, to which they readily agreed. The challenges for the deceased donor transplantation organization were whether to accept this VITT case as an organ donor, to identify which formalities were to be reported to the state and central authorities as adverse events following immunization (AEFI) before the donation process, and to identify if the recipients would have a chance of recurrence of thrombotic thrombocytopenia after transplantation.
After thorough consultation with the state and central vaccination authorities, appropriate documentation was done with respect to AEFI; after completing the legal formalities, the heart, lungs, liver, and 2 kidneys were retrieved. The postmortem examination was also performed by the forensic department from the government general hospital. The organs were allocated as per the regional allocation policy. Postmortem examination showed extensive thrombosis and hemorrhage in the brain but no evidence of disseminated intravascular coagulation in any of the visceral organs.
The heart and lung recipient was a 26-year-old woman who had been waiting on the list since June 2018 for heart and lung failure secondary to ventricular septal defect. She had a long waiting period owing to nonavailability of size-matched donor. Combined heart and lung transplant was done with informed consent. She had a smooth postoperative course and was extubated within 24 hours. She had a stable platelet count and did not face any untoward hematologic consequences.
The liver recipient was a 64-year-old woman with decompensate liver disease with no history of portal vein thrombosis or variceal bleed in the last 2 weeks and no history of vaccination in the last 4 weeks. Liver transplant was done after informed consent. She had an uneventful postoperative course except for mild elevation in D-dimer from 385 ng/dL (100-250 ng/mL) on day 1 of the postoperative period to a maximum of 3182 ng/dL (100-250 ng/mL) on day 8, and the nadir platelet count reported was 60,000/cumm (1,50,000-450,000/mm3) on day 7 postopertative . She received injection fondaparinus (Arixtra) 2.5 mg for a week followed by antiplatelet asprin 75 mg a day along with triple immunosuppression of prednisolone, tacrolimus and mycophenolate mofetil. Her liver function test normalized by day 5 and was discharged after 2 weeks.
The recipient of the first kidney was a 31-year-old woman who had been on the waiting list for 3 years with a diagnosis of presumed chronic interstitial nephritis and end-stage renal disease; she had been on maintenance hemodialysis for the past 4 years. Kidney transplantation was done after informed consent. She was given anti thymocyte globulin 3 mg/kg as induction and triple immunosuppression (prednisolone, tacrolimus and mycophenolate mofetil as maintenance therapy). She had immediate graft function with brisk diuresis. Her platelet count, D-dimer, and coagulation profile were normal throughout the postoperative period, and no hematologic complications were observed.
The recipient of the second kidney was a 22-year-old man with presumed chronic intestinal nephritis who had been on dialysis for 4 years and was waiting on a deceased donor list for 3 years. Kidney transplantation was done with informed consent, anti thymocyte globulin induction 3mg/kg, and triple immunosuppression. The postoperative course was uneventful with normal graft function and no hematologic complications.
These 6 donors with a median age of 48 y (37–72 y), 50% female, provided 20 organs to 17 recipients (Table (Table1).1). After a median follow-up of 43 d (19–93 d), all recipients were alive and 19 organs (95%) were functioning well. Two recipients (11.8%) developed thrombosis-related complications; 1 kidney developed thrombotic microangiopathy but recovered completely. One split-liver recipient developed thrombotic material in different vessels and necrosis of liver cells, resulting in urgent retransplantation.
Overall, the outcome of our small analysis of transplanted organs from possible VITT-related donors does not seem to differ from “non-VITT donors.” Nevertheless, caution is urged with these donors, and the acceptance of each organ remains to be assessed on an individual basis by the transplant team.
On April 4, we were offered a liver graft from a donation after brain death (DBD) donor with CVST and hepatic veins thrombosis occurred after ChAdOx1 nCov-19 vaccination.
The 32-y-old female received the first dose 11 d before hospitalization. Computed tomography (CT) highlighted CVST and hepatic vein thrombosis (Figure (Figure1).1). The donor presented thrombocytopenia with 35 000/mm3 platelets, 1.39 international normalized ratio, partial thromboplastin time of 36 s, elevated D-dimer of 120 800.0 μg/dL and hypofibrinogenemia with a value of 0.80 g/L, while liver function tests (LFTs) were normal.
Liver procurement followed the standard technique: the liver presented sharp margins without congestion; no biopsy was performed due to the excellent macroscopic aspect of the liver graft.
The recipient was a 69-y-old female with multifocal hepatocellular carcinoma and HCV-cirrhosis with model for end-stage liver disease score of 8.
LT followed the piggyback technique; survival outcomes following liver transplantation4 and balance of risk5 scores were 6 and 3, respectively.
Immunosuppression included induction by basiliximab, tapered steroids, and tacrolimus.
Postoperative course was uneventful with rapid graft recovery. With the aim to resolve or at least prevent any evolution of the hepatic venous thrombosis, a prophylactic dosage of 4000 IU/d low molecular weight heparin (LMWH) was started after surgery. A CT scan on POD10 showed complete thrombus resolution in the right and left hepatic vein tributaries (Figure (Figure1),1), and LMWH was therefore continued until discharge.
A woman in her 60s developed VITT 11 d after a first dose of ChAdOx1 nCov-19 vaccine (Oxford—Astra Zeneca). Onset was characterized by headache and behavioral disorders. Two days later, she was in a coma. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse-transcriptase polymerase chain reaction of a nasopharyngeal swab was negative. MRI disclosed cerebral venous sinus thrombosis (thrombosis of the left internal jugular vein, the sigmoid sinus, and the superior sagittal sinus), plus bilateral frontal hemorrhage and brain herniation. In addition, a thorax CT scan revealed a segmentary pulmonary embolism. Blood analysis revealed disseminated intravascular coagulation with severe thrombocytopenia, increased D-Dimer, prolonged activated partial thromboplastin time ratio and low fibrinogen. Anti-PF4 antibodies were incriminated in the pathophysiology of VITT 2 d later1: they were detected in our donor at a high titer using an enzyme-linked immunoassay, which detects the presence of anti-PF4/heparin antibodies (Stago Asserachrom Haem Polymerization Inhibitory Activity IgG): optical density was 2.17; n < 0.35. We confirmed their potency to activate platelets in a serotonin release assay, the gold standard functional method for heparin-induced thrombocytopenia diagnosis.5 The patient serum could activate normal platelets alone or with small amounts of heparin but not in the presence of high concentrations of heparin. A neurosurgical procedure was ruled out based on the patient’s poor neurological status at admission. She died within hours and was evaluated as an organ donor, under continuous hemodynamic noradrenaline support and with KDIGO stage 1 acute kidney injury.
The recipient of the heart and liver combined was a 63-y-old man with a restrictive cardiomyopathy and secondary cardiac cirrhosis. Cold ischemic times were 120 min for the heart and 510 min for the liver. Both organs were macroscopically normal before implantation. A standard unfractionated heparin anticoagulation was used during and after surgery. In addition to inotropic support, a temporary mechanical circulatory support by extracorporeal membrane oxygenation (ECMO) was required due to cardiac primary graft dysfunction. Echocardiographic monitoring showed a gradually recovering cardiac function, allowing the weaning of ECMO support at postoperative day (POD) 5 and of inotropic support at POD6. No postoperative bleeding or thrombotic complication occurred. At POD15, left ventricular ejection fraction was 60% and a protocol endomyocardial biopsy did not reveal any sign of acute allograft rejection or microvascular thrombosis. On the same day, liver biological tests were normal. A liver ultrasound showed a permeable hepatic artery and portal vein and no abnormality of the bile ducts.
The lung recipient was a 58-y-old woman with end-stage emphysema related to alpha-1 antitrypsin deficiency. A double lung transplantation was performed without extracorporeal circulatory support. The lungs were macroscopically normal. A thrombectomy was performed during the procedure. Ischemic time was 213 and 288 min for the left and right lungs, respectively. The recipient was extubated 4 h after surgery, required noninvasive ventilation until POD4, and was weaned off oxygen at POD6. Low-molecular-weight heparin anticoagulation was continued after ICU discharge at POD6. The first spirometry performed at POD15 showed normal lung function.
Kidneys were petechial before implantation. A biopsy of the right kidney performed before surgery revealed multiple thrombi within the glomerular capillaries of all 16 glomeruli except 2 devoid of thrombi (Figure 1). Arterioles were normal. The tubulointerstitial compartment displayed severe acute tubular injury. The recipient of the right kidney was a 70-y-old man with end-stage renal disease of undetermined origin. He was engrafted after 10 h of cold ischemia and recovered a renal function at POD4. Postoperative course was uneventful, and he was discharged at POD13.
We conclude that organs retrieved from the first French deceased donor victim of brain death after a VITT eventually recovered function after engraftment in 4 different recipients. However, renal recovery was laborious, and there was attendant glomerular scarring.
It’s behind a paywall & there’s no abstract
We identified 13 consented deceased organ donors, who presented with thrombosis and/or hemorrhage and laboratory features consistent with VITT, 4 between 28 January and 9 April 2021. All had received their first dose of ChAdOx1 nCoV‐19 vaccine before admission (see Table Table1).1). Ten donors proceeded to donate 27 allografts to 26 recipients. After a median follow‐up of 19 days, 21 of 27 (78%) allografts have satisfactory function. Three recipients developed early allograft failure requiring explantation (two livers and one kidney); two transplanted kidneys have impaired allograft function, currently requiring hemodialysis; and one recipient died within a day of transplantation from a presumed cardiac event. There were seven major thrombotic or hemorrhagic postoperative complications (three bleeds and four venous or arterial allograft thromboses) in six recipients, resulting in the loss of three transplants as described above; these events occurred within 9 days of transplantation. Of the six recipients with bleeding or thrombotic events, two had received their second dose of ChAdOx1 nCoV‐19 vaccine within 30 days before transplantation; neither patient had features suggestive of VITT at the time of transplantation. Two of the three patients with bleeding had preexisting risk factors for hemorrhage (dual antiplatelet agent therapy, anticoagulation for metallic cardiac valve); none of the patients with thromboses had significant preexisting procoagulant tendencies.
So far, three liver recipients had detectable anti‐PF4 antibodies between 3 and 22 days posttransplant; one of these recipients experienced a thrombotic complication without allograft loss and the other two had uncomplicated postoperative courses. Ten recipients (six kidneys and four livers) tested negative for anti‐PF4 antibodies.
The UK experience to date suggests that the potential risks of transplanting organs from donors with VITT are twofold. First, early major thrombosis or clinically significant bleeding, which may result from preexisting hemostatic and endothelial dysfunction in the allograft. Second, possible transmission of pathogenic lymphocytes producing anti‐PF4. The clinical significance of this is unclear; further follow‐up will determine whether this portends development of VITT in the recipient.
From April 9 up to May 7, 2021, we identified five potential deceased donors with VITT, using existing diagnostic criteria 1 with confirmation by hematologist evaluation. All the patients had received the ChAdOx1 n‐CoV‐19 adenoviral vector vaccine at a median of 13 days (range, 8–14 days) prior to intensive care unit admission. Four patients presented with cerebral venous thrombosis. All presented thrombosis in other sites, intracranial hemorrhage and thrombopenia (Table (Table1).1). Two organ procurement procedures were canceled (one due to family refusal and the other because of severe disseminated intravascular coagulation). Three organ procurement procedures were achieved. During these ones, two livers were discarded because of extensive portal vein thrombosis and one lung required a thrombectomy for segmental pulmonary embolism. Finally, 10 organs (six kidneys, two hearts, one lung, and one liver) were recovered and transplanted to nine recipients, who were followed until June 21, 2021 (Table (Table11).
After a median follow‐up of 52 days (range, 16–77 days), all recipients were alive with adequate functioning transplants. Patients experienced neither severe thrombotic nor hemorrhagic event. Two kidney transplant recipients displayed delayed graft function (including one showing glomerular microthrombi on preimplantation biopsy), that finally gradually recovered. One kidney transplant recipient underwent a biopsy at 10 days posttransplant showing mild interstitial inflammation and tubulitis, unrelated to VITT, which was treated by steroid pulses, with good subsequent function. Only one recipient displayed thrombocytopenia, but was present before transplantation and secondary to hepatic cirrhosis. To date, no recipient had detectable anti‐PF4 antibodies (tests performed at a median of 10 days posttransplant) and eight have been discharged from hospital (Table (Table11).
This report summarizes the outcomes of organ procurement and transplantation from deceased donors with VITT in France. The potential risks were to transplant organs with thrombosis (as in the case of disseminated intravascular coagulation 4 ), which could compromise organ function, and/or transmitting the systemic disease to the recipient. 5 To mitigate these risks, the French National Organ Procurement Agency immediately provided guidelines for the screening, procurement, and transplantation from such donors, as well as recipient monitoring (the detailed protocol is provided in the Supplementary Material). These guidelines led to the following: (1) a donation procedure was canceled in the case of severe disseminated intravascular coagulation; (2) organs were extensively assessed for the presence of thrombi (including biopsy if required) and discarded if thrombi were extensive; (3) after transplant, recipients underwent serial monitoring of thrombotic/hemorrhagic events, hemostasis, and anti‐PF4 antibodies. Our analysis shows that the early outcomes of these transplants were favorable. While the presence of thrombi in a few organs suggests the possibility that organ function could be compromised if thrombosis was extensive, we were able to select organs with limited thrombi and did not find any evidence of transmission of VITT to the recipients. In conclusion, our data support that organs from deceased donors with VITT may be suitable for transplantation and should be carefully assessed, but not systematically discarded.
Patient 2 was a 55-year-old healthy woman with no preexisting conditions except for mild hypothyroidism. She had never been treated with heparin. Seven days after receiving the first dose of ChAdOx1 nCoV-19 vaccine she started complaining of abdominal pain and presented to a first aid on the morning of day 10th. Routine blood examinations were normal except for the high level of D-dimer (5441 ng/mL) and mild thrombocytopenia (PLT 133,000). Abdominal ultrasound was normal. In the afternoon, during her hospital stay, she experienced a transient episode of aphasia and right hemiparesis, followed 2 h later by generalized seizures and coma. Orotracheal intubation was performed. Brain CT scan, angio-CT, and perfusion CT showed occlusion of the right internal carotid artery terminus and of the left MCA, extensive ischemic cores and severe bilateral hypoperfusion, without treatable penumbra (Fig. 3).
The patient was transferred to our ED. Blood examination repeated 10 h later showed worsening of thrombocytopenia (PLT 97,000 mm3) with a further decrease on the following day (PLT 59,000 mm3). Treatment with IVIG (1 g/kg per day) and dexamethasone 40 mg u.i.d. were started on day 1. TTE was normal. Twelve-hour post-stroke a total body CT revealed extensive portal vein thrombosis with occlusion of the left intrahepatic branches and left lower lobe subsegmental pulmonary arteries thrombosis. Brain CT scan showed bilateral malignant MCA infarct with uncal herniation (Fig. 3). Brain death was declared 24 h later. Autopsy was not performed due to consent denial by relatives.
A 33-year-old Korean man received the first dose of the ChAdOx1 nCoV-19 vaccination. He developed severe headache with vomiting 9 days after the vaccination. Twelve days after vaccination, he was admitted to the hospital with neurological symptoms and was diagnosed with cerebral venous sinus thrombosis, which was accompanied by intracranial hemorrhage. Thrombocytopenia and D-dimer elevation were observed, and the result of the PF4 enzyme-linked immunosorbent assay antibody test was reported to be strongly positive. Despite intensive treatment, including intravenous immunoglobulin injection and endovascular mechanical thrombectomy, the patient died 19 days after vaccination. Physicians need to be aware of thrombosis with thrombocytopenia syndrome (TTS) in adenoviral vector-vaccinated patients.
CASE DESCRIPTION: CA young woman without any medical history presented association of deep vein thrombosis and thrombocytopenia at day 10 after vaccine injection. The patient was treated with low-molecular weight heparin at a first medical institution. Twelve days post Ad26.COV2.S vaccination, the patient was admitted at our hospital for neurological deterioration and right hemiplegia. Medical imaging using MRI showed thrombosis of the major anterior part of the sagittal superior sinus with bilateral intraparenchymal hemorrhagic complications. Screening tests for antibodies against platelet factor 4 (PF4)-heparin by rapid lateral flow immunoassay and chemiluminescence techniques were negative. Platelet activation test using heparin-induced multiple electrode aggregometry confirmed the initial clinical hypothesis. Despite immediate treatment with intravenous immunoglobulin, dexamethasone, danaparoid and attempted neurosurgery the patient evolved toward brain death.
We present a case of brain death in a vaccinated, immunocompromised patient who presented with COVID-19 pneumonia. Imaging was characterized by diffuse cerebral edema, pseudo-subarachnoid hemorrhage, and no antegrade flow above the terminal internal carotid arteries. To our knowledge, this is the first case report with such findings in a vaccinated patient.
MRI of the brain performed the following day revealed acute left middle cerebral artery territory infarct, evolving right MCA territory infarcts, and redemonstration of previously seen parenchymal and subarachnoid blood products (Fig. 2 ). Contrast-enhanced MR venogram demonstrated non-occlusive superior sagittal sinus thrombosis (Fig. 3 ). After interdisciplinary discussion with hematology, neurosurgery and neurology a heparin drip was initiated following MR venogram confirmation of venous sinus thrombosis. On hospital day 19, head CT demonstrated continued expected evolution of infarcts with sulcal effacement surrounding the dominant left sided infarcts and trace rightward midline shift. Final head CT, performed on hospital day 35, demonstrated cessation of contrast opacification of the anterior and posterior intracranial arterial circulation as well as new cerebral edema with effacement of the gray-white matter borders, sulci, ventricles and cisterns, new hemorrhage in the right frontal and left frontoparietal lobes at the site of previous infarctions, and new hyperdensity in the sylvian fissures consistent with pseudo-subarachnoid hemorrhage (Fig. 4 ).
Here, we present a case of fatal rhabdomyolysis and compartment syndrome after ChAdOx1-nCoV-19 vaccination.
A 44-year-old previously healthy man developed generalized myalgia after his second dose of the adenoviral-based vaccine. He presented to ER with progressive weakness and brown urine two weeks later. On admission, his blood tests showed markedly elevated creatinine, creatine kinase, and severe metabolic acidosis. His bilateral forearm and gastrocnemius muscles were tense, tender and his skin was mottled. Computed tomography revealed bilateral retroperitoneal fluid collection and swelling and edematous change of his psoas muscles (Fig. 1 a).
The patient was promptly intubated and initiated on continuous venovenous hemofiltration because of severe metabolic acidosis and anuria. Emergent fasciotomy was performed over his four limbs to relieve pressure from acute compartment syndrome. Muscle biopsy revealed neutrophilic myositis and small-vessel vasculitis with fibrin thrombi and C4d deposition (Fig. 1b & c). The myositis panel was positive for anti-PM-Scl 100 autoantibodies. A heliotrope rash later developed over his eyelids. Dermatomyositis superimposed with rhabdomyolysis was impressed, and methylprednisolone (0.6 mg/kg/day) was given, followed by a pulsed dose of cyclophosphamide (3.6 mg/kg/day). In response to steroid therapy on day 7, his creatine kinase immediately peaked at 151058 U/L and declined rapidly thereafter (Fig. 1d). However, the limb wounds during 8-hourly wet dressing change became infected. Initial blood cultures on admission and on day 3 did not yield any pathogen. On day 5 of admission, his open fasciotomy wounds became contaminated with carbapenem-resistant Acinetobacter baumanii (CRAB) and despite maximal support and appropriate antibiotics, the patient died of CRAB sepsis and multiorgan failure on day 17.
Case report: We present a case of intracerebral haemorrhage after ChAdOx1-S vaccination. Middle-aged patient with no prior medical history was seen in the emergency room 16 days after the first dose of ChAdOx1-S vaccine with sudden onset left hemiplegia and severe holocranial oppressive headache. She did not receive heparin treatment in the previous 100 days. Blood test showed moderate thrombocytopenia and a right frontal lobar haemorrhage was seen on computed tomography scan, computed tomography venography was negative for thrombosis. The presence of antibodies against platelet factor 4 was confirmed. The patient's neurological condition progressively worsened. She developed a treatment resistant intracranial hypertension syndrome and she died three weeks later.
A 35-year-old woman with a medical history of migraine headaches and chronic thrombocytopenia presented to the emergency department (ED) with severe headache 7 days after vaccination with the J&J COVID-19 vaccine.
Fourty-eight hours after initial ED visit and 16 hours after discharge from the second ED visit, the patient experienced an acute loss of consciousness with an onset of generalized tonic-clonic seizure activity. On arrival to the ED, she was unresponsive with no purposeful movements. Neurologic examination revealed a Glasgow coma score of 3 with corneal, gag, and cough reflexes absent. Pupils were 6 mm and fixed. Noncontrast head CT revealed right temporal intraparenchymal hemorrhage and extensive subarachnoid hemorrhage (Figure 2A). There was also evidence of elevated intracranial pressure (sulci effacement) and resultant transtentorial herniation with obliteration of the basilar cisterns. A hyperdensity involving the superior sagittal sinus, right transverse sinus, and right sigmoid sinus suggested cerebral venous thrombosis (Figures 1C and 2B). Subsequent CT angiogram and venogram of the head revealed findings consistent with superior sagittal, transverse, and sigmoid sinus thrombosis (Figure 1D).
Laboratory evaluation revealed a platelet count of 10 K/uL, fibrinogen of 128 mg/dL, and D-dimer of 13476 ng/dL. Heparin-induced thrombocytopenia antibody assay was very elevated at 2.240 OD (normal <0.4). The patient had no exposure to heparin products. Evaluation by neurology and neurosurgery did not recommend any neurosurgical intervention secondary to futility. The patient had an ongoing absence of cortical activity with the absence of all cortical and brainstem reflexes. Nuclear medicine perfusion imaging documented the absence of cerebral perfusion.
The present report describes a vaccine-induced thrombotic thrombocytopenia (VITT) case with fatal exacerbation after initial improvement following initial intravenous immunoglobulin (IVIg) administration and anticoagulation. An 83-year-old woman presented at the emergency room with an alteration of her general condition. She presented with symptoms of weakness, nausea, vomiting, weight loss and spontaneous bruises without any obvious reason, 14 days after having received her first dose of ChadOx1 nCov-19.
During the night, she suffered from dyspnea grade NYHA 4. Pulmonary ventilation and perfusion (V/Q) scan was performed and disclosed bilateral pulmonary embolism. Anti-PF4 immunoglobulin G (IgG) antibodies (i.e. 1.80 AU/mL, Figure 1) were detected on day 1 postadmission using a PF4/polyvinylsulfonate rapid assay (HemosIL® AcuStar HIT IgG assay, Instrumentation Laboratory Belgium NV, Zaventem, Belgium). The diagnosis of VITT was confirmed using a heparin-induced multi-electrode aggregometry method.1
Unfortunately, the clinical status worsened on day 12 post-admission with a de novo reduction of platelet count. Abdominal CT scan showed right adrenal hematoma with left adrenal infiltrate, which is a usual presentation of adrenal infarction, as described in autoimmune heparin-induced thrombocytopenia and also recently in VITT.4,5 Four units of 500 IU/mL of prothrombin complex concentrate were administered on day 14 as an attempt to control the adrenal hematoma, but she died later that day from hypovolemic shock probably secondary to adrenal hemorrhage.
A 36-year-old female, known case of diabetes mellitus on oral therapy, presented to the emergency room with sudden onset of focal left-sided convulsions for 5 minutes followed by weakness in the left arm. She had a fever of 38.2 C° at home with vomiting and severe headache that started a few hours prior to presentation. Two weeks prior to the presentation, she received the first dose of the ChAdOx1 nCoV-19 vaccine. She had no history of any thromboembolic or connective tissue disorders.
She was started on enoxaparin 80 mg, antibiotics, and antivirals. Two hours later, the patient became hypotensive and tachycardic with a drop in GCS from 15 to 8 necessitating transfer to the Intensive Care Unit (ICU) where she was intubated, mechanically ventilated, and started on ionotropic support. One day later, the patient deteriorated as she developed florid DIC with a drop in hemoglobin to 4 g/dL requiring massive blood transfusion. A repeat blood work showed a highly elevated white blood cell count at 48.4×10"9/L (mainly neutrophils), low platelets at 94×10"9/L, low fibrinogen at 0.6 g/L, and high creatinine at 254 umol/L. She developed acute kidney injury and severe acidosis requiring emergent hemodialysis. Enoxaparin was stopped due to DIC. A repeat brain CT scan showed multiple new bifrontal and biparietal hypodensites. The CT-venogram demonstrated extensive dural venous sinus thrombosis of the superior sagittal sinus and its cortical tributaries as well as the proximal left transverse sinus (Fig. 2 ). CT abdomen and pelvis showed extensive portal vein thrombosis with superior mesenteric vein thrombosis and potential splenic and hepatic infarction. The patient had a complicated course in the ICU given her multiple thromboses, DIC, difficulty to use anticoagulants, lactic acidosis, acute kidney injury, and multi-organ failure. She developed cardiac arrest with pulseless electrical activity and died four days after admission.
A 78‐year‐old woman with no personal or family history of abnormal bleeding noticed a bruise on her thigh 2 weeks after her second COVID‐19 (SARS‐CoV‐2) mRNA vaccination (Pfizer‐BioNTech; day 1; not shown). Otherwise, she had no serious adverse events following vaccination. Her skin bruise persisted. On day 38, a new skin bruise appeared on her left hand. On day 50, she visited a hospital because of pain and swelling in the left hand (Figure 1A). She had no evidence of any malignancies or autoimmune disorders and no medication intake. Her SARS‐CoV‐2 antigen was negative. The hematoma was removed to treat her compartment syndrome of the left hand. However, she had persistent postoperative bleeding and red blood cell units were transfused (2 units/day for 3 days). On day 55, she was transferred to our hematology department.
Her physical examination revealed ecchymosis on her hind upper arms and left knee (not shown). Computed tomography (CT) scans showed intra‐articular bleeding in her left shoulder (Figure 1B) and asymptomatic central nervous system (CNS) bleeding (Figure 1C). Accordingly, the patient received 2 units of fresh frozen plasma for 4 days with no apparent effect.
Blood tests revealed normocytic anemia (red cell count 2.40 × 106/μL, reference range 3.86–4.92 × 106/μL; hemoglobin 7.8 g/dL, 11.6–14.8 g/dL; hematocrit 22.8%, 35.1%–44.4%) with normal platelet count (159 × 103/μL, 158–348 × 103/μL), prothrombin time (10.9 s, 9.5–13.5 s), and activated partial thromboplastin time (25.9 s, 25.0–38.0 s). Fibrinogen level was 288 mg/dL (155–415 mg/dL). Factor VIII/8 (F8) activity was >201%, (62%–145%), von Willebrand factor (VWF) activity 241% (50%–150%), and its antigen level was >201% (50%–150%). F8 inhibitor was negative. Hepatitis B surface antigen, Hepatitis C antibody, and HIV‐1 and ‐2 antibodies were negative or nonreactive. No monoclonal protein was detected by initial screening.
While factor XIII/13 (F13) antigen level was slightly reduced (59%, reference range >70%), its activity was below the detection limit (<3%, 70%–140%; measured by an ammonia release assay using a Berichrom FXIII Kit (Sysmex Corporation, Kobe, Japan) at a commercial laboratory service (SRL Ltd., Hachioji, Japan). Administration of F13 concentrates (1200 units/day for 5 days) significantly improved her symptoms, and she was discharged home on day 74. In an experimental work‐up conducted by the Japanese collaborative research group for autoimmune coagulation factor deficiencies (AiCFD), a 5‐step dilution mixing test with a healthy control plasma showed an F13 inhibitor pattern (Figure 1E) and anti‐F13‐A subunit autoantibodies were detected by immunochromatography and enzyme‐liked immunosorbent assay (Figure 1G,H, respectively). Therefore, we made a definite diagnosis of autoimmune F13 deficiency (AiF13D), according to the ISTH/SSC criterion 2015. 1 Immediately, prednisolone (0.5 mg/kg daily) was administered orally on day 91, but she noticed a new bruise on her right hand on day 100. Since F13 activity was still low (14%), she was re‐hospitalized on day 103. Screening CT did not detect any internal bleeding. Prednisolone was increased to 1 mg/kg, and intravenous (IV) immunoglobulin (IVIg, 400 mg/kg for 5 days) was administered. However, she was found lying on the floor in the early morning on day 109. She had a mild disturbance of consciousness but did not complain of headache. She had no elevated blood pressure, vomiting, paralysis, or other local neurological deficits. She was given 1200 units of F13 concentrates and her F13 activity increased to 33%; however, she died of CNS bleeding (i.e., cerebral hemorrhage and subarachnoid hemorrhage) in the afternoon on the same day after approximately 10 h (Figure 1D). Since the patient's family refused to autopsy, the exact cause and/or trigger of CNS bleedings remained unknown.
During the maintenance phase, the patient underwent anti-SARS-CoV-2 vaccination (mRNA vaccine BNT162b29), with the first, second, and third vaccine administrations given on 15 April, 6 May, and 27 December 2021, respectively.
On 3 January 2022, a week after the third vaccine dose, the patient developed mild symptoms characterized by low-grade fever and cough for which he underwent a nasal/oro-pharyngeal swab test for SARS-CoV-2, which was positive.
However, from the same day of the SARS-CoV-2 negative test, the patient experienced high-grade fever (39 °C) with dyspnea. On January 24 he started antibiotic therapy with ceftriaxone without any clinical benefit. In the same day, a chest X-ray showed infiltrates of parenchymal thickening in the right middle and lower lobe. On January 31, he tested negative for SARS-CoV-2 on the molecular swab once again.
Starting from 8 February, the patient began to experience dyspnea with oxygen desaturation. On February 10, he tested again negative for SARS-CoV-2 on the molecular test PCR.
On February 13 he was put on a high flow nasal cannula (HFNC), 50% FiO2, 60 L/min, due to severe respiratory failure (Pa02/FiO2 ratio of 163 from the arterial blood gas analysis).
Due to the worsening respiratory symptoms, a chest CT scan and fibrobronchoscopy were obtained. The CT scan showed large areas of bilateral and symmetrical ground glass with crazy paving pattern, without, however, opacification defects of the pulmonary arteries (Supplementary Figure S1). Furthermore, considering the clinical and radiological picture, a further swab for the search for SARS-CoV-2 was analyzed and at this time was positive with the threshold cycle (CT) of the RT-PCR of 27 for ORF 1ab and 28 for N and for E genes. That same day, SARS-CoV-2-antigen was detected in the serum at high load (178 pg/mL) by chemiluminescent enzyme immunoassay (Lumipulse-G SARS-CoV-2-Ag, Fujirebio Holdings, Tokyo, Japan). Moreover, the specimen collected during the fibrobronchoscopy was negative for tuberculosis and common bacterial culture, but positive for SARS-CoV-2-RNA.
Despite the HFNC, the patient presented desaturations and was therefore placed in a helmet with continuous positive airway pressure (CPAP), 40% FiO2, and positive end expiratory pressure (PEEP) equal to 8 cm H2O. Moreover, a blood test showed total immunoglobulins IgG and IgM of 194 mg/dL (range 800–1800) and <19.2 mg/dL (range 80–280), respectively, and persistence of an inflammatory state characterized by CRP 170 mg/L, procalcitonin 0.30 μg/mL, fibrinogen 542 mg/dL, and D-dimer 5016 μg/mL.
Due to progressive respiratory failure (Pa02/FiO2 ratio of 85 at arterial blood gas analysis), on February 16 he was transferred to the intensive care unit (ICU), where he underwent orotracheal intubation and received, while pending the results of the microbiological tests performed during the fibrobronchoscopy, broad-spectrum antibiotics, anti-Pneumocystis jirovecii and herpes-virus prophylaxis, corticosteroid, and tocilizumab treatment. On March 4, the COVID-19 surveillance swab was still positive. After a few days of ICU stay, he developed several infectious complications, and, subsequently, a septic shock by mechanical ventilation-associated pneumonia, which led to death on 6 March.
An 85-year-old man presented with vertigo and vomiting one day after receiving the second dose of the BNT162b2 vaccine (Fig. 1A).
The vertigo and vomiting improved gradually, and the patient was discharged from the hospital on the 3rd day after vaccination. Subsequently, he presented with progressive gait disturbance and urinary retention, and he was admitted to the hospital because of gait disturbance on the 15th post-vaccination day. He had no fever or other symptoms suggestive of infection.
Brain CT and MRI were unremarkable, however, on the 16th day after vaccination, thoracic MRI revealed a longitudinal hyperintense lesion from the Th3–5 vertebral levels on T2-weighted imaging (Fig. 1B and C). On the 25th day after vaccination, the patient was referred to our hospital. Routine physical examination and RT-PCR for COVID-19 were normal. He was bedridden and neurological examination revealed that he was disoriented, with right-dominant paraplegia. He also had hypoesthesia in both lower limbs and hyporeflexia in both his upper and lower limbs, without an extensor plantar response. His consciousness immediately improved the following day. Blood tests revealed elevated levels of HbA1c (6.9%) and C-reactive protein (CRP) (3.26 mg/dL). Serologic tests confirmed the absence of anti-aquaporin (AQP)-4 antibodies. Cerebrospinal fluid (CSF) analysis demonstrated predominantly monomorphonuclear pleocytosis (11 cells/μL), with elevated protein levels (120 mg/dL) and the absence of oligoclonal bands. The IgG index (0.67) and myelin basic protein (MBP) level (58.1 pg/mL) were normal. Bacterial cultures and CSF cytology results were unremarkable. The patient was diagnosed with ATM, and intravenous methylprednisolone (0.5 g) for three days, followed by oral prednisolone (40 mg/day), was administered from the 30th day after vaccination. However, the patient's neurological symptoms did not improve. A second course of intravenous methylprednisolone (0.5 g) for three days from the 42nd day after vaccination still did not resolve his neurological symptoms. On the 52nd day after vaccination, he presented with fever and oxygen desaturation. Blood tests revealed pancytopenia with an elevated CRP level (15.72 mg/dL). Chest radiography revealed bilateral pneumonia. Treatment with intravenous sulbactam/ampicillin (6 g/day) did not improve the patient's condition, and on the 58th day after vaccination, he died. His family declined a postmortem examination.
A 55-year-old Kenyan male, diagnosed in the past with generalized MG, diabetes, and hypertension presented to the emergency room (ER) with breathlessness, chest pain, profuse sweating, and cold clammy extremities.
Two weeks after taking the first dose of the ChAdOx1-S (recombinant) vaccine (AstraZeneca batch number 210157; AstraZeneca plc, Cambridge, United Kingdom), the patient reported dysphagia, dysarthria, and breathlessness for a further period of three weeks during which the condition of the patient deteriorated as the symptoms worsened. The frequency of pyridostigmine was increased to every two hourly (12 times a day) upon instruction by the neurologist. However, this did not reverse the condition.
Due to difficulty in breathing, the patient was immediately intubated and admitted to the intensive care unit (ICU) for observation and treatment of a myasthenic crisis with concomitant myocardial infarction. The patient was started on enoxaparin sodium 60 mg twice daily for eight days.
After 24 hours, the patient was extubated successfully; however, the dysphagia and dysarthria persisted. During this time the patient developed severe diarrhea characterized by a tremendous decline in blood pressure and tidal volumes. A blood-gas analysis revealed slight compensated metabolic acidosis accompanied by an increased level of glucose (Table 3).
The septic screen was negative. Re-intubation was performed and a dopamine infusion (1 microgram per kilogram per minute) was started in conjunction with intravenous fluids (4L of normal saline 24 hourly) as a response to an ejection fraction of 28%, low blood pressure, and tachycardia, all of which tended towards cardiogenic shock. Five days later, the patient was weaned off the dopamine infusion. Extubation was again attempted but was unsuccessful. The patient was started on prednisone 30 mg tablet once daily and pyridostigmine 60 mg tablet two hourly. Intravenous immunoglobulin (IVIG) infusion 35 g daily for five days was also commenced. Over a span of the next two weeks, the condition remained unchanged. However, after this time period, the patient developed an event of an ST-elevated myocardial infarction (STEMI). This further deteriorated the patient’s condition, which was manifested by severe lethargy and flaccidity. A percutaneous tracheostomy was performed in an attempt to wean off the ventilator. The patient later developed a fever of 38.8°C and a decline in blood pressure and oxygen saturation (80%). A repeat chest x-ray showed opacification bilaterally in the lung base, which was consistent with a pneumonia-like picture. At this stage, a repeat RT-PCR was performed, which was still negative. Intravenous meropenem 1g thrice a day was initiated to treat the infection. A few days later, the patient developed septic shock characterized by a blood pressure of 70/37 mmHg and the blood culture showed, the multi-drug resistant Acinetobacter baumannii but sensitive to piperacillin/tazobactam, which was administered every six hours (4.5g). However, the patient sustained three further myocardial infarctions the next day leading to death.
Herein, we describe clinical records of a 63-year woman with fulminant myocarditis following ChAdOx1 nCoV-19 vaccination that was salvaged by heart transplantation. She complained chest pain, nausea, vomiting, and fever after the second vaccination. After the heart transplantation, the patient died due to necrotizing pneumonia on the 54th day of onset. Fulminant myocarditis is very rare after ChAdOx1 nCoV-19 vaccination but can be fatal.
Patient 1, a 35-year-old Asian woman, developed episodic right temporal and periorbital headache 6 days after receiving the ChAdOx1 nCoV-19 vaccine. Five days later, she awoke with left face, arm and leg weakness, right gaze preference and drowsiness. Non-contrast CT and CT angiography (CTA) revealed occlusion of the right middle cerebral artery (MCA) distal M1 segment with extensive ischaemia and haemorrhagic transformation (figure 1A-C)). Subsequent imaging revealed right portal vein thrombosis. The platelet count was 64 x 109/L (reference range 150 –400 x 109/L); D-dimer was raised at 11 220 µg/L (reference range 0–550); and the Asserachrom HPIA IgG assay for anti-PF4 antibodies was positive (76.1%). The patient underwent urgent decompressive hemicraniectomy (figure 1D). The platelet count increased after intravenous immune globulin and plasmapheresis. She received anticoagulation with intermediate dose fondaparinux. Fourteen days after presentation, her conscious level suddenly dropped; CT head showed extensive haemorrhagic transformation of the left MCA infarct with mass effect and herniation of the brain through the decompressive hemicraniectomy. Brainstem death was subsequently confirmed.
A 57-year-old man, who was previously healthy and did not receive medication, was admitted after a four-day history of intense headache. On arrival, the patient was hypertensive (with a blood pressure of 165/87 mmHg), bradycardic (sinus rhythm, frequency 37 beats/min), uncontactable, Glascow coma score 6 with light rigid pupils and anisocoria. Blood tests showed thrombocytopenia (16 × 109/l), elevated fibrin D-dimer (> 20 FEU mg/l) and normal international normalized ratio (INR). A CT of the cerebrum (CTc) was performed, which showed significant right-sided intracerebral hemorrhage (ICH) with midline shift ( Figure 1). 2 g of tranexamic acid was given intravenously. Intubation and neuroprotective therapy were initiated with sedation (propofol/sufentanil), relaxation (rocuronium), hypertonic saline (2 mmol/kg), elevated headrest and ensured mean arterial pressure > 80 mmHg.
The patient was transferred to a neurosurgical center. On arrival two hours after admission to the main hospital, the patient was respiratory and circulatory stable without the use of vasopressor/inotropy. Biochemically, there was worsening of thrombocytopenia (9 × 109/l), fibrinogen level was low (1.7 μmol/l) and INR was slightly increased (1.3) with normal activated partial thromboplastin time (26 s). Three portions of platelet concentrate were given under rapid infusion. Thrombobelastography (TEG) had an R time of 12.3 s (time to clot formation), clot strength was 5.2 mm, and fibrinogen contribution to clot strength was 2 mm. The parameters were compatible with a hypocoagulable state. The patient was assessed to be clinically incarcerated; CTc showed progression of ICH and an angiogram was free of aneurysms/malformations. Flow cytometry was seen to be without acute promyelocystic leukaemia, this was examined in relation to differential diagnosis. The treatment was completed two and a half hours after the initial admission, as further treatment was deemed hopeless.
Twelve days before admission, the patient had been vaccinated with ChAdOx1 nCoV-19. Due to the symptomatology and the absence of medical history, the patient's condition was suspected to be a side effect of the vaccination. Analysis of heparin PF 4 was not carried out-Ab (IgG) on plasma via Greinacher's laboratory in Germany. Enzyme immunoassay for VITT was not yet set up. Acute heparin-induced thrombocytopenia (HIT) analysis was performed on blood drawn during platelet transfusion. The analysis had been refrigerated for two days. It was thus agreed, in cooperation with the laboratory manager, to interpret the result as inconclusive due to platelet infusion, because the sample had been delayed, and because new independent samples did not manage to be sent to VITT diagnostics in Germany. Treatment with intravenous immunoglobulin (IVIG) could have been initiated, but due to the rapid progression and because the event occurred prior to current guidelines, the patient did not receive this treatment.
The first case highlights a patient with rapid decline into coma within a few hours of arrival, despite having normal CSF analysis and minimal involvement of the brainstem on initial brain MRI. Benign CSF findings are not atypical in ADEM, with normal CSF leukocyte counts and protein levels reported in about half of patients (16). MRI abnormalities may be minimal or absent during the first 2 weeks of ADEM presentation (17). The rapid clinical decline can be attributed to increased brainstem involvement that became more evident on subsequent neuroimaging. ADEM subgroups presenting with rapid decline in consciousness are associated with higher mortality rates (18).


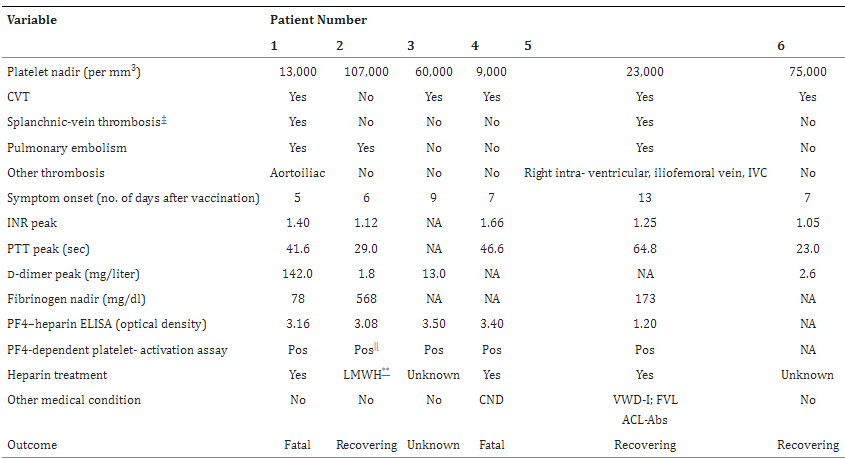
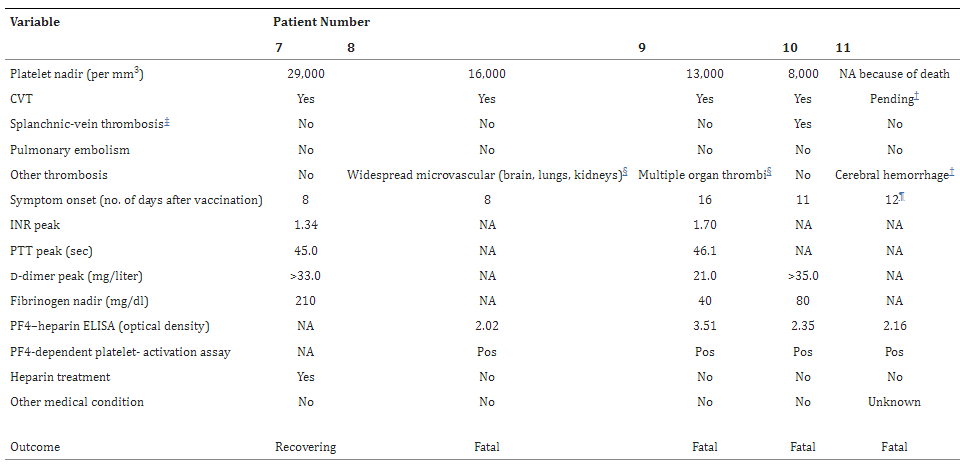


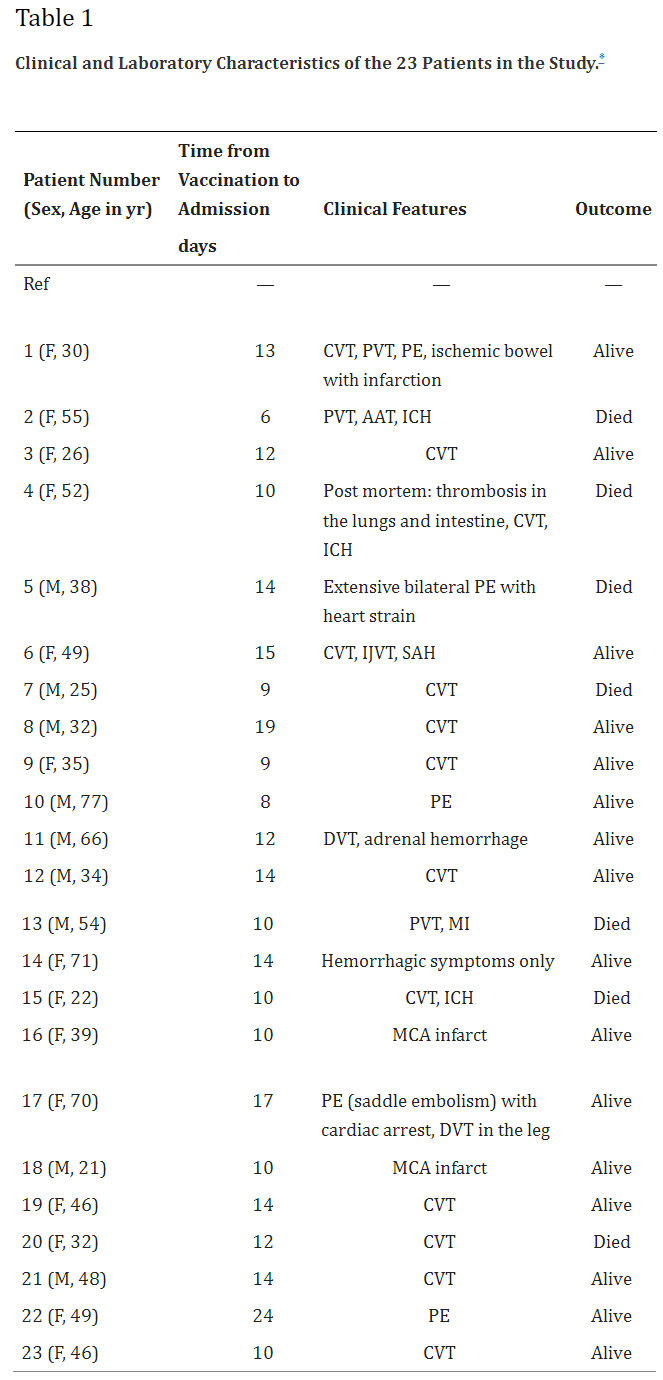
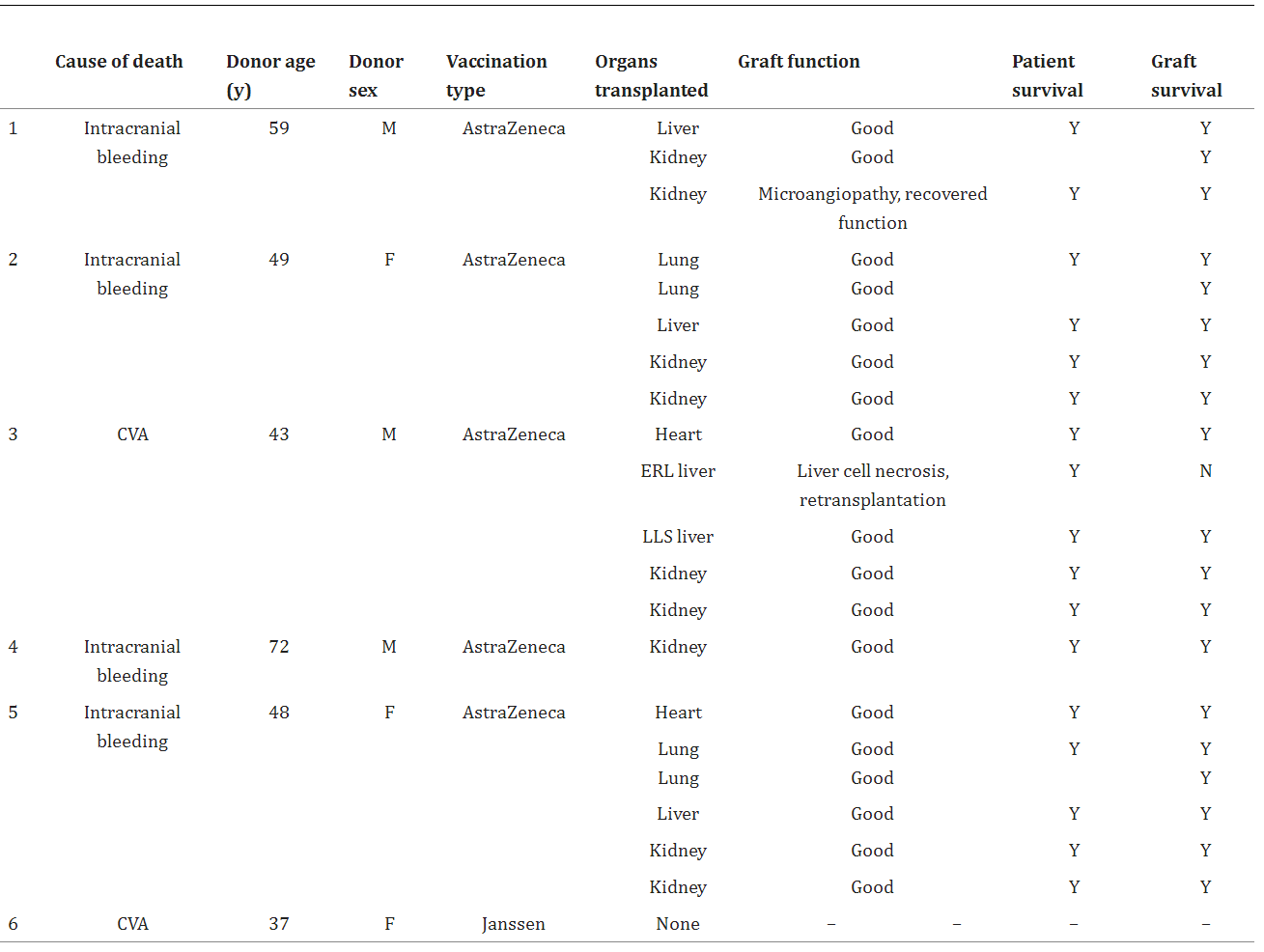



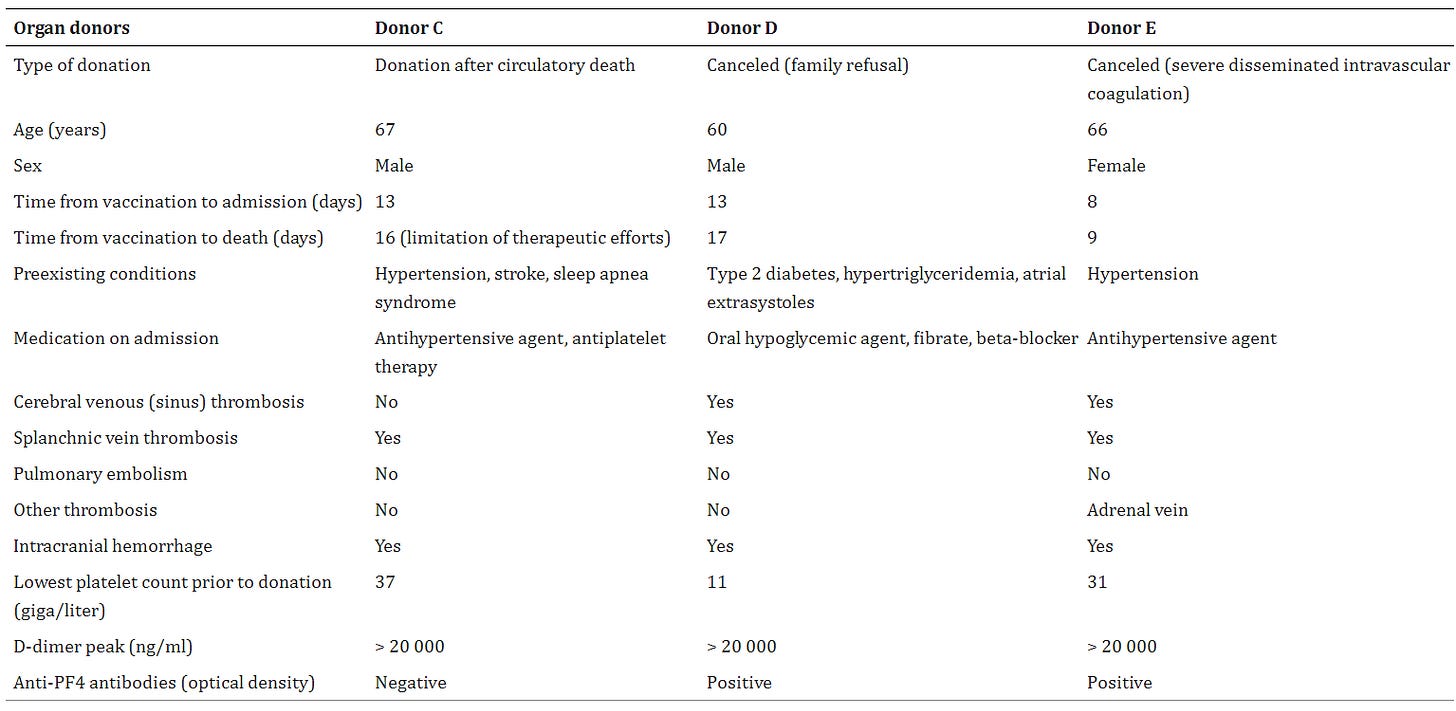
Remarkable accomplishment. Thank you so much for this excellent documentation. Let us hope that Dr. Rand Paul will gain access to these documents and present them in the new Congress.  dr. Fauci should be forced to explain in front of the American people. 
Fascinating, thank you.