List of Studies Relating to Vaccine/Infection/Cross-Reactive/Innate Immunities
This is the list used by Daniel Horowitz to write his article on the avalanche of immunity studies
In order to properly utilize this resource, it is necessary to bear in mind a few pointers:
· This is designed to highlight only research supportive of the strength and superiority of non-vaccine immunities. Therefore, I have to some degree deliberately not included studies bereft of any data supporting my positions. Anyone who wishes to see such studies can go to the CDC/NIH/etc websites.
· Just because a study’s author/s say something in the study does not make it true, or even make it a warranted conclusion or inference from the study’s data. Very often, we find studies where the authors include language that in our opinion is basically deceptive. This is typically done either to satisfy the diktats of editors and other individuals who wield influence over the medical journals (who will refuse to carry a paper without the appropriate disclaimers such as “of course we should all still wear masks” and the like), or because they personally believe that their results, if published without some additional ‘helpful interpretation’, will sow confusion and hesitancy in a segment of the public regarding the “consensus” public health policy choices. I deliberately avoided quoting (to some degree) statements by study authors that I judged to be incorrect or unwarranted.
· Many studies often are disingenuously named, or contain relevant findings nestled deep within the study’s “discussion” or supplementary materials. A few of the cited studies herein were included for having such “incidental” findings or data, and my quotes from such studies therefore may actually misrepresent what the study claims as its primary finding/s, since they are intended to highlight a specific fact or data point from the study while not granting credibility to the rest of it.
Re Vax & Infection Immunity:
1. Discrete Immune Response Signature to SARS-CoV-2 mRNA Vaccination Versus Infection
While both infection and vaccination induced robust innate and adaptive immune responses, our analysis revealed significant qualitative differences between the two types of immune challenges. In COVID-19 patients, immune responses were characterized by a highly augmented interferon response which was largely absent in vaccine recipients. Increased interferon signaling likely contributed to the observed dramatic upregulation of cytotoxic genes in the peripheral T cells and innate-like lymphocytes in patients but not in immunized subjects. Analysis of B and T cell receptor repertoires revealed that while the majority of clonal B and T cells in COVID-19 patients were effector cells, in vaccine recipients clonally expanded cells were primarily circulating memory cells. Importantly, the divergence in immune subsets engaged, the transcriptional differences in key immune populations, and the differences in maturation of adaptive immune cells revealed by our analysis have far-ranging implications for immunity to this novel pathogen.
Results Among the 52238 included employees, 1359 (53%) of 2579 previously infected subjects remained unvaccinated, compared with 22777 (41%) of 49659 not previously infected. The cumulative incidence of SARS-CoV-2 infection remained almost zero among previously infected unvaccinated subjects, previously infected subjects who were vaccinated, and previously uninfected subjects who were vaccinated, compared with a steady increase in cumulative incidence among previously uninfected subjects who remained unvaccinated. Not one of the 1359 previously infected subjects who remained unvaccinated had a SARS-CoV-2 infection over the duration of the study. In a Cox proportional hazards regression model, after adjusting for the phase of the epidemic, vaccination was associated with a significantly lower risk of SARS-CoV-2 infection among those not previously infected (HR 0.031, 95% CI 0.015 to 0.061) but not among those previously infected (HR 0.313, 95% CI 0 to Infinity).
Conclusions Individuals who have had SARS-CoV-2 infection are unlikely to benefit from COVID-19 vaccination, and vaccines can be safely prioritized to those who have not been infected before.
Conclusion Natural infection resulting in detectable anti-spike antibodies and two vaccine doses both provide robust protection against SARS-CoV-2 infection, including against the B.1.1.7 variant.
Viral loads were higher, i.e., Ct values lower, in symptomatic infections (median [IQR] Ct 16.3 [13.5-21.7]) compared to asymptomatic screening (20 [14.5-29.5]) (Figure 4A, Kruskal-Wallis p<0.001).Unvaccinated seronegative HCWs had the highest viral loads (Ct: 18.3 [14.0-25.5]), followed by vaccinated previously seronegative HCWs (Ct: 19.7 [15.0-27.5]); unvaccinated seropositive HCWs had the lowest viral loads.
There was an unexpected rise in incidence above baseline levels in the first two weeks following vaccination, which remained to some extent after adjustment (Figure 3B).
Further, the Beta strain was neutralized equally well by Natural Infection 2020 and 2021 sera, with NAb titers (235.03 [95% CI 171.15-322.76] and 306.53 [95% CI 223-421.35], respectively) that were higher than that elicited by CoronaVac (NTGM 35.03 [95% CI 27.46-44.68]), differing by 6.71 [4.10-11.0], and 8.75 [5.34-14.3] folds, respectively. Similarly, the Delta strain was neutralized equally well by Natural Infection 2020 and 2021 sera, but with markedly lower NAb titers than those obtained with the Beta strain (NTGM 69.15 [95% CI 50.35-94.96] and NTGM 94.35 [95% CI 68.64-129.69], respectively), and CoronaVac’s NAb titers were even lower still (NTGM 24.48 [95% CI 19.2 -31.23]; being 2.82 [1.73-4.62], and 3.85 [2.35-6.32] folds lower in comparison, respectively), almost at the limit of detection (Figure 2B). Together, these results highlight the relatively low NAb titers elicited by CoronaVac compared to natural infection.
The SARS-CoV-2 Delta variant and its sublineages (B.1.617.2, AY.1, AY.2, AY.3; [1]) can cause high viral loads, are highly transmissible, and contain mutations that confer partial immune escape [2,3]. Using PCR threshold cycle (Ct) data from a single large contract laboratory, we show that individuals in Wisconsin, USA had similar viral loads in nasal swabs, irrespective of vaccine status, during a time of high and increasing prevalence of the Delta variant. Infectious SARS-CoV-2 was isolated from 51 of 55 specimens (93%) with Ct <25 from both vaccinated and unvaccinated persons, indicating that most individuals with Ct values in this range (Wilson 95% CI 83%-97%) shed infectious virus regardless of vaccine status. Notably, 68% of individuals infected despite vaccination tested positive with Ct <25, including at least 8 who were asymptomatic at the time of testing. Our data substantiate the idea that vaccinated individuals who become infected with the Delta variant may have the potential to transmit SARS-CoV-2 to others. Vaccinated individuals should continue to wear face coverings in indoor and congregate settings, while also being tested for SARS-CoV-2 if they are exposed or experience COVID-like symptoms.
6. Single cell profiling of T and B cell repertoires following SARS-CoV-2 mRNA vaccine | bioRxiv
Natural infection induced expansion of larger CD8 T cell clones occupied distinct clusters, likely due to the recognition of a broader set of viral epitopes presented by the virus not seen in the mRNA vaccine.
In infection-naïve individuals, the second dose boosted the quantity but not quality of the T cell response, while in convalescents the second dose helped neither. Spike-specific T cells from convalescent vaccinees differed strikingly from those of infection-naïve vaccinees, with phenotypic features suggesting superior long-term persistence and ability to home to the respiratory tract including the nasopharynx.
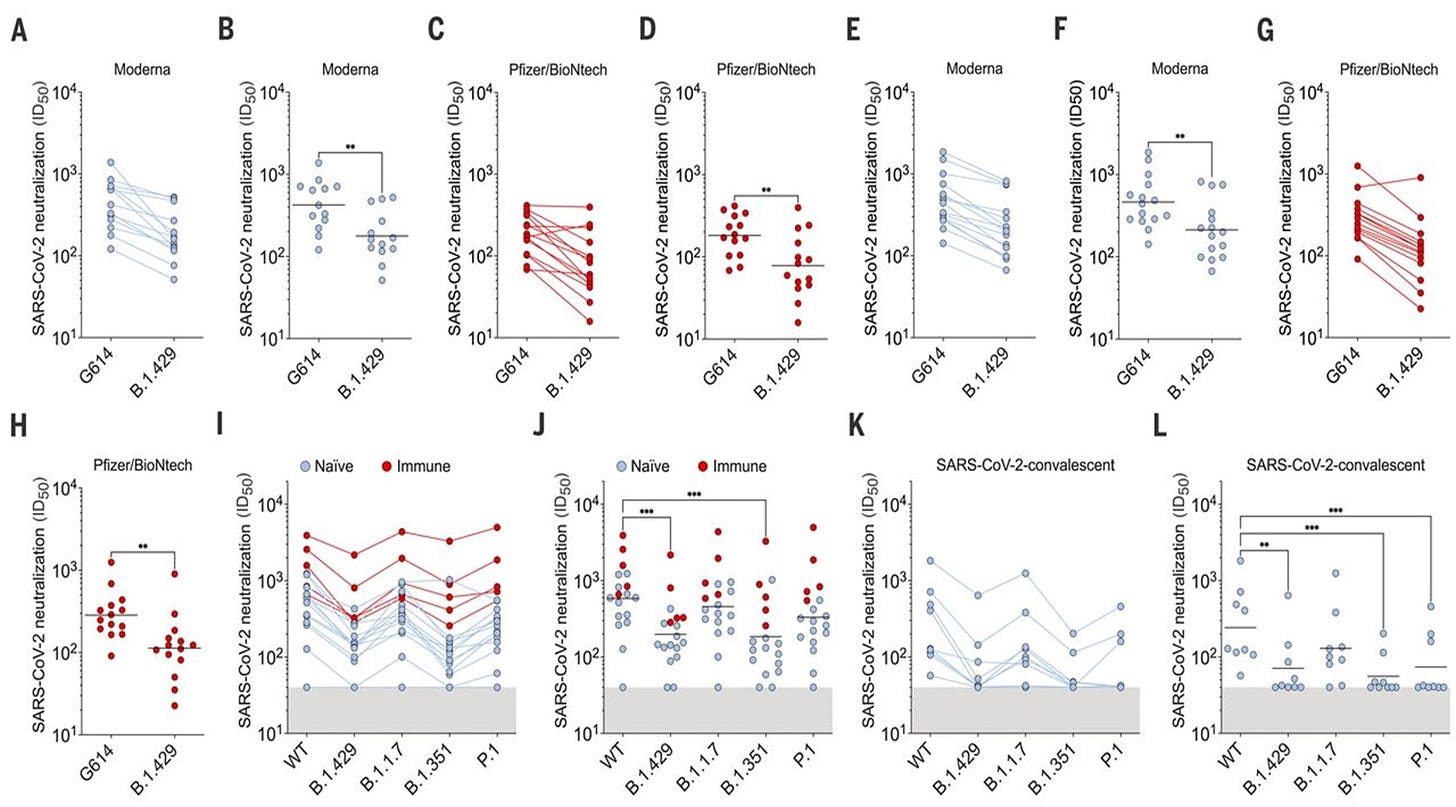
8. SARS-CoV-2 immune evasion by the B.1.427/B.1.429 variant of concern | Science
After one dose, individuals with prior infection showed enhanced T cell immunity, antibody-secreting memory B cell response to the spike protein, and neutralizing antibodies effective against variants B.1.1.7 and B.1.351. By comparison, HCW receiving one vaccine dose without prior infection showed reduced immunity against variants. B.1.1.7 and B.1.351 spike mutations resulted in increased, abrogated, or unchanged T cell responses, depending on human leukocyte antigen (HLA) polymorphisms.
SARS-CoV-2 naïve individuals, but not recovered individuals, received an additional boost to antigen-specific CD4+ T cells following the second vaccine dose (Fig. 1B). Overall, mRNA vaccination induced a universal CD4+ T cell response, as all individuals, regardless of prior infection with SARS-CoV-2, had greater frequencies of AIM+ CD4+ T cells post-boost than at baseline (Fig. S1D).
AIM+ CD8+ T cells had a similar subset distribution to AIM+ CD4+ T cells. Total non-naïve CD8+ T cells were distributed throughout memory T cell subsets and the frequencies of these subsets were unchanged by vaccination (Fig S2B). The baseline antigen-specific CD8+ T cell response in recovered subjects was composed of similar proportions of EM1, CM, and terminally-differentiated CD8+ EMRA (CD45RA+ CD27− CCR7−) T cells (Fig. 2C-D). A smaller proportion of AIM+ EM2 and EM3 CD8+ T cells was observed at baseline in recovered subjects. These proportions stayed relatively consistent throughout the course of vaccination in recovered subjects, and there were no statistically significant changes from baseline (Fig. 2D). In contrast, in SARS-CoV-2 naive individuals, few AIM+ EMRA CD8+ T cells were observed at any time point (Fig. 2D). Rather, vaccine-primed AIM+ CD8+ T cells in these subjects were largely EM1 with minority populations of CM and EM3 cells (Fig. 2D).
The second vaccination dose appears to exert a detrimental effect in the overall magnitude of the spike-specific humoral response in COVID-19 recovered individuals;
The second BNT162b2 vaccine dose results in a reduction of cellular immunity in COVID-19 recovered individuals, which suggests that a second dose, according to the current standard regimen of vaccination, may be not necessary in individuals previously infected with SARS-CoV-2.
Conclusions and Relevance Prior COVID-19 infection but not ongoing Long-COVID symptoms were associated with an increase in the risk of self-reported adverse events following BNT162b2/Pfizer vaccination. COVID-19 illness-vaccination interval did not significantly influence AEs.
Results Survey was completed by 2,002 respondents, of whom 26.6% had prior COVID-19 infection (68.8% laboratory confirmed). Prior COVID-19 infection was associated with increased risk of any side effect (risk ratio 1.08, 95% confidence intervals [1.05-1.11]), fever (2.24 [1.86-2.70]), breathlessness (2.05 [1.28-3.29]), flu-like illness (1.78 [1.51-2.10]), fatigue (1.34 [1.20-1.49]) and local reactions (1.10 [1.06-1.15]). It was also associated with increased risk of severe side effects, leading to hospital care (1.56 [1.14-2.12]).
Vaccination was highly effective with overall estimated efficacy for documented infection of 92·8% (CI:[92·6, 93·0]); hospitalization 94·2% (CI:[93·6, 94·7]); severe illness 94·4% (CI:[93·6, 95·0]); and death 93·7% (CI:[92·5, 94·7]). Similarly, the overall estimated level of protection from prior SARS-CoV-2 infection for documented infection is 94·8% (CI:[94·4, 95·1]); hospitalization 94·1% (CI:[91·9, 95·7]); and severe illness 96·4% (CI:[92·5, 98·3]). Our results question the need to vaccinate previously-infected individuals.
16. Antibody Evolution after SARS-CoV-2 mRNA Vaccination | bioRxiv
Between prime and boost, memory B cells produce antibodies that evolve increased neutralizing activity, but there is no further increase in potency or breadth thereafter. Instead, memory B cells that emerge 5 months after vaccination of naïve individuals express antibodies that are equivalent to those that dominate the initial response. We conclude that memory antibodies selected over time by natural infection have greater potency and breadth than antibodies elicited by vaccination. These results suggest that boosting vaccinated individuals with currently available mRNA vaccines would produce a quantitative increase in plasma neutralizing activity but not the qualitative advantage against variants obtained by vaccinating convalescent individuals.
17. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization | Nature
In individuals who had not previously been infected with SARS-CoV-2, a single dose of either the Pfizer or the AstraZeneca vaccine induced a barely detectable level of neutralizing antibodies against the Delta variant. About 10% of the sera neutralized this variant. However, a two-dose regimen generated high sero-neutralization levels against the Alpha, Beta and Delta variants in individuals sampled at week 8 to week 16 after vaccination.
Our results demonstrate that the emerging Delta variant partially—but notably—escapes neutralizing monoclonal antibodies and polyclonal antibodies elicited by previous infection with SARS-CoV-2 or by vaccination.
18. Neutralization of VOCs including Delta one year post COVID-19 or vaccine
Interpretation Persistent neutralization of the wide-spread Alpha and Delta variants one year after wild-type infection may aid vaccine policy makers in low-resource settings when prioritizing vaccine supply. The reduced capacity of neutralizing Beta and Gamma strains, but not the Alpha and Delta strains following both infection and three different vaccine regimens argues for caution against Beta and Gamma-exclusive mutations in the efforts to optimize next generation SARS-CoV-2 vaccines.
20. Cross-reactive CD4+ T cells enhance SARS-CoV-2 immune responses upon infection and vaccination
Abstract
While evidence for pre-existing SARS-CoV-2-cross-reactive CD4+ T cells in unexposed individuals is increasing, their functional significance remains unclear. Here, we comprehensively determined SARS-CoV-2-cross-reactivity and human coronavirus-reactivity in unexposed individuals. SARS-CoV-2-cross-reactive CD4+ T cells were ubiquitous, but their presence decreased with age. Within the spike glycoprotein fusion domain, we identified a universal immunodominant coronavirus-specific peptide epitope (iCope). Pre-existing spike- and iCope-reactive memory T cells were efficiently recruited into mild SARS-CoV-2 infections and their abundance correlated with higher IgG titers. Importantly, the cells were also reactivated after primary BNT162b2 COVID-19 mRNA vaccination in which their kinetics resembled that of secondary immune responses. Our results highlight the functional importance of pre-existing spike-cross-reactive T cells in SARS-CoV-2 infection and vaccination.
Results No significant difference was observed between the 20B and 19A isolates for HCWs with mild COVID-19 and critical patients. However, a significant decrease in neutralisation ability was found for 20I/501Y.V1 in comparison with 19A isolate for critical patients and HCWs 6-months post infection. Concerning 20H/501Y.V2, all populations had a significant reduction in neutralising antibody titres in comparison with the 19A isolate. Interestingly, a significant difference in neutralisation capacity was observed for vaccinated HCWs between the two variants whereas it was not significant for the convalescent groups.
Conclusion Neutralisation capacity was slightly reduced for critical patients and HCWs 6-months post infection. No neutralisation escape could be feared concerning the two variants of concern in both populations. The reduced neutralising response observed towards the 20H/501Y.V2 in comparison with the 19A and 20I/501Y.V1 isolates in fully immunized subjects with the BNT162b2 vaccine is a striking finding of the study.
Summary Natural infection resulting in detectable anti-spike antibodies and two vaccine doses both provided ≥ 85% protection against symptomatic and asymptomatic SARS-CoV-2 infection in healthcare workers, including against the B.1.1.7 variant. Single dose vaccination reduced symptomatic infection by 67%.
Most important, we found a relative reduction in the reactivity of the sera with the B.1.1.7 versus the Wuhan-1 variant after the second boosting immunization. These data allow to make a comparison of different vaccines in terms of anti-S antibody generation and cast doubts about the convenience of repeatedly immunizing with the same S protein sequence.
Although antibodies made in response to vaccines based on the original Wuhan-1 strain sequence do also bind the B.1.1.7 variant, suggesting that most epitopes are conserved between both sequences, there is a relative loss of reactivity with the B.1.1.7 variant compared to the Wuhan-1 strain occurring upon administration of the booster dose of vaccine. This is somehow expected since repeated immunization with the same antigen sequence leads to the generation of higher affinity antibodies that fit better the epitopes of the immunogen. This increase in affinity has the negative side effect of reducing the “breadth” of the antibodies, that is, their capacity to bind to epitopes that differ slightly from those of the immunogen. Such effect has been observed before, for instance, as a result of vaccination with inactivated influenza virus isolated in immunization campaigns, that result in an efficient neutralization of that particular seasonal variant of influenza but results in reduced capacity to neutralize other variants14. By contrast, antibody responses to natural infection are broad and exhibit different immunodominance patterns14. Another example of the different breadth in the antibody responses elicited by natural infection versus vaccination is the long-term struggle, and still unsuccessful, to generate a vaccine that prevents infection by the tremendous diversity of clades and mutants of human immunodeficiency virus (HIV), with the general conclusion that the tested vaccines are effective to prevent infection by the strain used for immunization but not by the myriad of variants found in the field15. In regard to COVID-19, a broad and sustained polyantigenic immunoreactivity against the S protein and other viral proteins has also been found in COVID-19 patients, in this case associated to the severity of symptoms16. Our follow-up of the cohort of residents in nursing homes that were previously naturally infected with SARS-CoV-2 and later vaccinated with two doses of BNT/Pfizer shows that the first dose of vaccine increased the already existing titer of anti-S antibodies against the Wuhan-1 strain. However, the second dose did not result in further increases in the antibody titer and had the negative counterpart of reducing the relative reactivity of the antibodies with the B-1-1-7 VOC. Indeed, such loss of breadth was already detected as soon as 15 days after the first dose of vaccine, when the antibody titer against the Wuhan-1 strain had not yet increased significantly.
Results: A total of 2,653 individuals fully vaccinated by two doses of vaccine during the study period and 4,361 convalescent patients were included. Higher SARS-CoV-2 IgG antibody titers were observed in vaccinated individuals (median 1581 AU/mL IQR [533.8-5644.6]) after the second vaccination, than in convalescent individuals (median 355.3 AU/mL IQR [141.2-998.7]; p<0.001). In vaccinated subjects, antibody titers decreased by up to 40% each subsequent month while in convalescents they decreased by less than 5% per month. Six months after BNT162b2 vaccination 16.1% subjects had antibody levels below the seropositivity threshold of <50 AU/mL, while only 10.8% of convalescent patients were below <50 AU/mL threshold after 9 months from SARS-CoV-2 infection. Conclusions: This study demonstrates individuals who received the Pfizer-BioNTech mRNA vaccine have different kinetics of antibody levels compared to patients who had been infected with the SARS-CoV-2 virus, with higher initial levels but a much faster exponential decrease in the first group.
Adults who have been fully vaccinated against SARS-CoV-2 can carry the same viral load of the delta variant as those who are unvaccinated, a preliminary analysis of UK data suggests.1
Results: SARS-CoV-2-naive vaccinees had a 13.06-fold (95% CI, 8.08 to 21.11) increased risk for breakthrough infection with the Delta variant compared to those previously infected, when the first event (infection or vaccination) occurred during January and February of 2021. The increased risk was significant (P<0.001) for symptomatic disease as well. When allowing the infection to occur at any time before vaccination (from March 2020 to February 2021), evidence of waning natural immunity was demonstrated, though SARS-CoV-2 naive vaccinees had a 5.96-fold (95% CI, 4.85 to 7.33) increased risk for breakthrough infection and a 7.13-fold (95% CI, 5.51 to 9.21) increased risk for symptomatic disease. SARS-CoV-2-naive vaccinees were also at a greater risk for COVID-19-related-hospitalizations compared to those that were previously infected. Conclusions: This study demonstrated that natural immunity confers longer lasting and stronger protection against infection, symptomatic disease and hospitalization caused by the Delta variant of SARS-CoV-2, compared to the BNT162b2 two-dose vaccine-induced immunity.
Highlights
Measuring immunity to SARS-CoV-2 is key for understanding COVID-19 and vaccine development
Epitope pools detect CD4+ and CD8+ T cells in 100% and 70% of convalescent COVID patients
T cell responses are focused not only on spike but also on M, N, and other ORFs
T cell reactivity to SARS-CoV-2 epitopes is also detected in non-exposed individuals
Understanding adaptive immunity to SARS-CoV-2 is important for vaccine development, interpreting coronavirus disease 2019 (COVID-19) pathogenesis, and calibration of pandemic control measures. Using HLA class I and II predicted peptide “megapools,” circulating SARS-CoV-2-specific CD8+ and CD4+ T cells were identified in ∼70% and 100% of COVID-19 convalescent patients, respectively. CD4+ T cell responses to spike, the main target of most vaccine efforts, were robust and correlated with the magnitude of the anti-SARS-CoV-2 IgG and IgA titers. The M, spike, and N proteins each accounted for 11%–27% of the total CD4+ response, with additional responses commonly targeting nsp3, nsp4, ORF3a, and ORF8, among others. For CD8+ T cells, spike and M were recognized, with at least eight SARS-CoV-2 ORFs targeted. Importantly, we detected SARS-CoV-2-reactive CD4+ T cells in ∼40%–60% of unexposed individuals, suggesting cross-reactive T cell recognition between circulating “common cold” coronaviruses and SARS-CoV-2.
An important question is arising as COVID-19 vaccines are getting rolled out: Should individuals who already had a SARS-CoV-2 infection receive one or two shots of the currently authorized mRNA vaccines. In this short report, we show that the antibody response to the first vaccine dose in individuals with pre-existing immunity is equal to or even exceeds the titers found in naïve individuals after the second dose. We also show that the reactogenicity is significantly higher in individuals who have been infected with SARS-CoV-2 in the past. Changing the policy to give these individuals only one dose of vaccine would not negatively impact on their antibody titers, spare them from unnecessary pain and free up many urgently needed vaccine doses.
28. Shedding of Infectious SARS-CoV-2 Despite Vaccination | medRxiv
Abstract
The SARS-CoV-2 Delta variant might cause high viral loads, is highly transmissible, and contains mutations that confer partial immune escape 1,2. Outbreak investigations suggest that vaccinated persons can spread Delta 3,4. We compared RT-PCR cycle threshold (Ct) data from 699 swab specimens collected in Wisconsin 29 June through 31 July 2021 and tested with a qualitative assay by a single contract laboratory. Specimens came from residents of 36 counties, most in southern and southeastern Wisconsin, and 81% of cases were not associated with an outbreak. During this time, estimated prevalence of Delta variants in Wisconsin increased from 69% to over 95%. Vaccination status was determined via self-reporting and state immunization records.
Main text
We observed low Ct values (<25) in 212 of 310 fully vaccinated (68%; Figure 1A) and 246 of 389 (63%) unvaccinated individuals. Testing a subset of low-Ct samples revealed infectious SARS-CoV-2 in 15 of 17 specimens (88%) from unvaccinated individuals and 37 of 39 (95%) from vaccinated people (Figure 1B).
Low Ct values were detected in vaccinated people regardless of symptoms at the time of testing (Figure 1C). Ct values <25 were detected in 7 of 24 unvaccinated (29%; CI: 13-51%) and 9 of 11 fully vaccinated asymptomatic individuals (82%; CI: 48-97%), and 158 of 232 unvaccinated (68%, CI: 62-74%) and 156 of 225 fully vaccinated (69%; CI: 63-75%) symptomatic individuals. Time from symptom onset to testing did not vary by vaccination status (p=0.40; Supplemental Figure 2). Infectious virus was detected in the sole specimen tested from an asymptomatic fully vaccinated individual. Although few asymptomatic individuals were sampled, these results indicate that even asymptomatic, fully vaccinated people might shed infectious virus.
Combined with other studies 2–5, these data indicate that vaccinated and unvaccinated individuals infected with the Delta variant might transmit infection. Importantly, we show that infectious SARS-CoV-2 is frequently found even in vaccinated persons when specimen Ct values are low. The inclusion of viruses from Pango lineages B.1.617.2, AY.2, and AY.3, and multiple counties without a linking outbreak, indicate that Delta-lineage SARS-CoV-2 can achieve low Ct values consistent with transmissibility in fully vaccinated individuals across a range of settings. Vaccinated and unvaccinated persons should get tested when symptomatic or after close contact with someone with suspected or confirmed COVID-19. Continued adherence to non-pharmaceutical interventions during periods of high community transmission to mitigate spread of COVID-19 remain important for both vaccinated and unvaccinated individuals.
The calculated attack rate among all exposed patients and staff was 10.6% (16/151) for staff and 23.7% (23/97) for patients, in a population with 96.2% vaccination rate (238 vaccinated/248 exposed individuals).
Among the patients (median age: 77 years; range: 42–93; median time from second vaccine dose to infection: 176 days; range: 143-188), eight became severely ill, six critically ill and five of the critically ill died. (All of the unvaccinated cases were described as mild, even though one of them was in his 80s.)
30. The BNT162b2 mRNA vaccine against SARS-CoV-2 reprograms both adaptive and innate immune responses
Interestingly, however, the BNT162b2 vaccine also modulated the production of inflammatory cytokines by innate immune cells upon stimulation with both specific (SARS-CoV-2) and non-specific (viral, fungal and bacterial) stimuli. The response of innate immune cells to TLR4 and TLR7/8 ligands was lower after BNT162b2 vaccination, while fungi-induced cytokine responses were stronger. In conclusion, the mRNA BNT162b2 vaccine induces complex functional reprogramming of innate immune responses, which should be considered in the development and use of this new class of vaccines.
31. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar
32. Breakthrough SARS-CoV-2 infections in 620,000 U.S. Veterans, February 1, 2021 to August 13, 2021
For the period February 1, 2021 – August 13, 2021, full vaccination with either Pfizer-BioNTech (aHR 0.16, 95% CI 0.16, 0.17) or Moderna (aHR: 0.13, 95% CI 0.12, 0.13) was associated with lower risk of infection. For the period mid-March 2021 – August 13, 2021, full vaccination with Janssen was also associated with a lower risk of infection (aHR 0.30, 95% CI 0.28, 0.32), owing to the fact that full vaccination with the Janssen vaccine was not possible until March because of timing of authorization. However, these protective associations declined over time (p<0.01 for time dependence, Table 2), even after adjusting for age and comorbidity. The proportionate reduction in infection associated with vaccination declined for all vaccine types, with the largest declines for Janssen followed by Pfizer-BioNTech and Moderna (Figure 1).Specifically, in March, protection against infection was: 88% (95% CI, 87% to 89%) for Janssen; 92% (95% CI, 92% to 93%) for Moderna; and 91% (95% CI, 91% to 92%) for Pfizer-BioNTech. By August, protection against infection had declined to: 3% (95% CI, -7% to 12%) for Janssen; 64% (95% CI, 62%-66%) for Moderna; and 50% (95% CI, 47% to 52%) for Pfizer-BioNTech.
Between Dec 14, 2020, and Aug 8, 2021, of 4 920 549 individuals assessed for eligibility, we included 3 436 957 (median age 45 years [IQR 29–61]; 1 799 395 [52·4%] female and 1 637 394 [47·6%] male). For fully vaccinated individuals, effectiveness against SARS-CoV-2 infections was 73% (95% CI 72–74) and against COVID-19-related hospital admissions was 90% (89–92). Effectiveness against infections declined from 88% (95% CI 86–89) during the first month after full vaccination to 47% (43–51) after 5 months. Among sequenced infections, vaccine effectiveness against infections of the delta variant was high during the first month after full vaccination (93% [95% CI 85–97]) but declined to 53% [39–65] after 4 months. Effectiveness against other (non-delta) variants the first month after full vaccination was also high at 97% (95% CI 95–99), but waned to 67% (45–80) at 4–5 months. Vaccine effectiveness against hospital admissions for infections with the delta variant for all ages was high overall (93% [95% CI 84–96]) up to 6 months.
34. COVID-19 vaccine surveillance report Week 42
N antibody levels appear to be lower in individuals who acquire infection following 2 doses of vaccination
35. The Nucleocapsid protein triggers the main humoral immune response in COVID-19 patients
Most of the COVID-19 patients presented a specific immune response against the full length and fragments of the N protein and, to lesser extent, against a fragment containing amino acids 300–685 of the S protein. In contrast, immunoreactivity against other S protein fragments or the M protein was low. This response is specific for COVID-19 patients as very few of the control patients displayed immunoreactivity, likely reflecting an immune response against other coronaviruses.
36. Ultrapotent antibodies against diverse and highly transmissible SARS-CoV-2 variants
RESULTS
Blood from 22 convalescent subjects who recovered from SARS-CoV-2 WA-1 infection was screened for neutralizing and binding activity, and four subjects with high reactivity against the WA-1 variant were selected for antibody isolation. SARS-CoV-2 spike (S)–reactive antibodies were identified through B cell sorting with S protein–based probes. WA-1 live-virus neutralization assays identified four RBD-targeting antibodies with high potency [half-maximal inhibitory concentration (IC50) 2.1 to 4.8 ng/ml], two of which were derived from the same IGHV1-58 germline but from different donors. Antigen-binding fragments (Fabs) of these antibodies exhibited nanomolar affinity to S (2.3 to 7.3 nM). Competition assays and electron microscopy indicated that two of the most potent antibodies blocked angiotensin-converting enzyme 2 (ACE2) and bound open conformation RBD, whereas the other two bound both up and down conformations of RBD and blocked ACE2 binding. Binding and lentivirus neutralization assays against 13 circulating VOCs or variants of interest—including B.1.1.7, B.1.351, B.1.427, B.1.429, B.1.526, P.1, P.2, B.1.617.1, and B.1.617.2—indicated that these antibodies were highly potent against VOCs despite being isolated from subjects infected with early ancestral SARS-CoV-2 viruses. Cryo-EM studies of the two most potent antibodies in complex with S revealed that these antibodies target a site of vulnerability on RBD but have minimal contacts with mutational hotspots, defining the structural basis for their high effectiveness against the emerging VOCs and further delineating an IGHV1-58 antibody supersite. To investigate potential mechanisms of escape, we applied antibody selection pressure to replication-competent vesicular stomatitis virus (rcVSV) expressing the WA-1 SARS-CoV-2 S (rcVSV-SARS2) and identified S mutations that conferred in vitro resistance. We evaluated these antibodies individually or in combinations for their capacity to prevent rcVSV-SARS2 escape and discovered that antibody combinations with complementary modes of recognition to the RBD lowered the risk of resistance.
CONCLUSION
Our study demonstrates that convalescent subjects previously infected with ancestral variant SARS-CoV-2 produce antibodies that cross-neutralize emerging VOCs with high potency. Structural and functional analyses reveal that antibody breadth is mediated by targeting a site of vulnerability at the RBD tip offset from major mutational hotspots in VOCs. Selective boosting of immune responses targeting specific RBD epitopes, such as the sites defined by these antibodies, may induce breadth against current and future VOCs.
People who contracted COVID-19 had a similar viral load regardless of whether they had been vaccinated. The study further found that 25 percent of vaccinated household contacts contracted COVID-19. while 38 percent of unvaccinated individuals were diagnosed with the disease.
Vaccinated individuals with prior infection 6 months or more before dose 1 had statistically significantly lower risk for breakthrough infection than those vaccinated less than 6 months before dose 1 (adjusted hazard ratio, 0.62 [95% CI, 0.42-0.92]; P = .02 for BNT162b2 and 0.40 [95% CI, 0.18-0.91]; P = .03 for mRNA-1273 vaccination). (Translation: vaccine might be ruining the development of immunity from infection.)
Conclusions and relevance: Prior SARS-CoV-2 infection was associated with a statistically significantly lower risk for breakthrough infection among individuals receiving the BNT162b2 or mRNA-1273 vaccines in Qatar between December 21, 2020, and September 19, 2021.
Re ADE/Variants:
2. COVID-19 Vaccines: Should We Fear ADE? | The Journal of Infectious Diseases | Oxford Academic
Abstract
Might COVID-19 vaccines sensitize humans to antibody-dependent enhanced (ADE) breakthrough infections? This is unlikely because coronavirus diseases in humans lack the clinical, epidemiological, biological, or pathological attributes of ADE disease exemplified by dengue viruses (DENV). In contrast to DENV, SARS and MERS CoVs predominantly infect respiratory epithelium, not macrophages. Severe disease centers on older persons with preexisting conditions and not infants or individuals with previous coronavirus infections. Live virus challenge of animals given SARS or MERS vaccines resulted in vaccine hypersensitivity reactions (VAH), similar to those in humans given inactivated measles or respiratory syncytial virus vaccines. Safe and effective COVID-19 vaccines must avoid VAH.
Conclusions
These SARS-CoV vaccines all induced antibody and protection against infection with SARS-CoV. However, challenge of mice given any of the vaccines led to occurrence of Th2-type immunopathology suggesting hypersensitivity to SARS-CoV components was induced. Caution in proceeding to application of a SARS-CoV vaccine in humans is indicated.
However, in the case of the Delta variant, neutralizing antibodies have a decreased affinity for the spike protein, whereas facilitating antibodies display a strikingly increased affinity. Thus, ADE may be a concern for people receiving vaccines based on the original Wuhan strain spike sequence (either mRNA or viral vectors). Under these circumstances, second generation vaccines with spike protein formulations lacking structurally-conserved ADE-related epitopes should be considered.
5. Out of the frying pan and into the fire? Due diligence warranted for ADE in COVID-19
Abstract
Antibody-dependent enhancement (ADE) is an atypical immunological paradox commonly associated with dengue virus re-infection. However, various research models have demonstrated this phenomenon with other viral families, including Coronaviridae. Recently, ADE in SARS-CoV-2 has emerged as one hypothesis to explain severe clinical manifestations. Whether SARS-CoV-2 is augmented by ADE remains undetermined and has therefore garnered criticism for the improper attribution of the phenomenon to the pandemic. Thus, critical evaluation of ADE in SARS-CoV-2 vaccine development will be indispensable to avoid a global setback and the erosion of public trust.
Lessons from RSV vaccines
There have been warnings that ADE should be fully evaluated for coronavirus vaccines to avoid repeating the tragic failure of the RSV vaccine28. The first RSV vaccine, based on formalin-inactivated RSV (FI-RSV), entered a clinical trial in 1965, a time when several other inactivated or attenuated virus-based vaccines had already been successfully developed, such as vaccines against smallpox29 and polio30. The FI-RSV vaccine was well tolerated and appeared to be moderately immunogenic at first. However, instead of protecting study participants, the FI-RSV vaccine exhibited a paradoxical disease-strengthening effect (enhanced respiratory disease (ERD); Box 1) during subsequent natural RSV infection. Among the 20 infants who received the FI-RSV vaccine, 16 required hospitalization, including two who subsequently died, whereas only one of the 21 participants in the control group was hospitalized31. The FDA then urgently suspended all clinical studies of RSV vaccines.
7. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies
Antibody-based drugs and vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being expedited through preclinical and clinical development. Data from the study of SARS-CoV and other respiratory viruses suggest that anti-SARS-CoV-2 antibodies could exacerbate COVID-19 through antibody-dependent enhancement (ADE). Previous respiratory syncytial virus and dengue virus vaccine studies revealed human clinical safety risks related to ADE, resulting in failed vaccine trials. Here, we describe key ADE mechanisms and discuss mitigation strategies for SARS-CoV-2 vaccines and therapies in development. We also outline recently published data to evaluate the risks and opportunities for antibody-based protection against SARS-CoV-2.
We find no difference in viral loads when comparing unvaccinated individuals to those who have vaccine “breakthrough” infections. Furthermore, individuals with vaccine breakthrough infections frequently test positive with viral loads consistent with the ability to shed infectious viruses. Our results, while preliminary, suggest that if vaccinated individuals become infected with the delta variant, they may be sources of SARS-CoV-2 transmission to others.
9. Correlation of SARS-CoV-2 Breakthrough Infections to Time-from-vaccine; Preliminary Study | medRxiv
A nationwide vaccination campaign was initiated early in Israel, allowing for a real-world evaluation of the interaction between protection and time-from-vaccine. The Delta (B.1.617.2) variant became the dominant strain in Israel in June 2021, as Israel is currently experiencing a new surge of cases. Leveraging the centralized computerized database of Maccabi Healthcare Services (MHS), we assessed the correlation between time-from-vaccine and incidence of breakthrough infection. We found that the risk for infection was significantly higher for early vaccinees compared to those vaccinated later. This preliminary finding should prompt further investigagions into long-term protection against different strains, and prospective clinical trials to examine the effect of a booster vaccine against breakthrough infection.
Cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) acquisition after vaccination with BNT162b2 have been described, but the risk of secondary transmission from fully vaccinated individuals remains ill defined. Herein we report a confirmed transmission of SARS-CoV-2 alpha variant (B.1.1.7) from a symptomatic immunocompetent woman 4 weeks after her second dose of BNT162b2, despite antispike seroconversion.
PCR cycle threshold (Ct) values were similar between both vaccinated and unvaccinated groups at diagnosis, but viral loads decreased faster in vaccinated individuals. Early, robust boosting of anti-spike protein antibodies was observed in vaccinated patients, however, these titers were significantly lower against B.1.617.2 as compared with the wildtype vaccine strain.
12. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort
In this large population study of patients tested for SARS-CoV-2 by RT-PCR following two doses of mRNA BNT162b2 vaccine, we observe a significant increase of the risk of infection in individuals who received their last vaccine dose since at least 146 days ago, particularly among patients older than 60.
13. Transmission of SARS-CoV-2 Delta Variant Among Vaccinated Healthcare Workers, Vietnam
Interpretation: Breakthrough Delta variant infections are associated with high viral loads, prolonged PCR positivity, and low levels of vaccine-induced neutralizing antibodies, explaining the transmission between the vaccinated people.
The spike protein receptor-binding domain (RBD) of SARS-CoV-2 is the molecular target for many vaccines and antibody-based prophylactics aimed at bringing COVID-19 under control. Such a narrow molecular focus raises the specter of viral immune evasion as a potential failure mode for these biomedical interventions. With the emergence of new strains of SARS-CoV-2 with altered transmissibility and immune evasion potential, a critical question is this: how easily can the virus escape neutralizing antibodies (nAbs) targeting the spike RBD? To answer this question, we combined an analysis of the RBD structure-function with an evolutionary modeling framework. Our structure-function analysis revealed that epitopes for RBD-targeting nAbs overlap one another substantially and can be evaded by escape mutants with ACE2 affinities comparable to the wild type, that are observed in sequence surveillance data and infect cells in vitro. This suggests that the fitness cost of nAb-evading mutations is low. We then used evolutionary modeling to predict the frequency of immune escape before and after the widespread presence of nAbs due to vaccines, passive immunization or natural immunity. Our modeling suggests that SARS-CoV-2 mutants with one or two mildly deleterious mutations are expected to exist in high numbers due to neutral genetic variation, and consequently resistance to vaccines or other prophylactics that rely on one or two antibodies for protection can develop quickly -and repeatedly- under positive selection. Predicted resistance timelines are comparable to those of the decay kinetics of nAbs raised against vaccinal or natural antigens, raising a second potential mechanism for loss of immunity in the population. Strategies for viral elimination should therefore be diversified across molecular targets and therapeutic modalities.
In July, vaccine effectiveness against hospitalization has remained high (mRNA-1273: 81%, 95% CI: 33-96.3%; BNT162b2: 75%, 95% CI: 24-93.9%), but effectiveness against infection was lower for both vaccines (mRNA-1273: 76%, 95% CI: 58-87%; BNT162b2: 42%, 95% CI: 13-62%), with a more pronounced reduction for BNT162b2. Notably, the Delta variant prevalence in Minnesota increased from 0.7% in May to over 70% in July whereas the Alpha variant prevalence decreased from 85% to 13% over the same time period. Comparing rates of infection between matched individuals fully vaccinated with mRNA-1273 versus BNT162b2 across Mayo Clinic Health System sites in multiple states (Minnesota, Wisconsin, Arizona, Florida, and Iowa), mRNA-1273 conferred a two-fold risk reduction against breakthrough infection compared to BNT162b2 (IRR = 0.50, 95% CI: 0.39-0.64). In Florida, which is currently experiencing its largest COVID-19 surge to date, the risk of infection in July after full vaccination with mRNA-1273 was about 60% lower than after full vaccination with BNT162b2 (IRR: 0.39, 95% CI: 0.24-0.62).
mRNA-based vaccines provide effective protection against most common SARS-CoV-2 variants. However, identifying likely breakthrough variants is critical for future vaccine development. Here, we found that the Delta variant completely escaped from anti-N-terminal domain (NTD) neutralizing antibodies, while increasing responsiveness to anti-NTD infectivity-enhancing antibodies. Although Pfizer-BioNTech BNT162b2-immune sera neutralized the Delta variant, when four common mutations were introduced into the receptor binding domain (RBD) of the Delta variant (Delta 4+), some BNT162b2-immune sera lost neutralizing activity and enhanced the infectivity. Unique mutations in the Delta NTD were involved in the enhanced infectivity by the BNT162b2-immune sera. Sera of mice immunized by Delta spike, but not wild-type spike, consistently neutralized the Delta 4+ variant without enhancing infectivity. Given the fact that a Delta variant with three similar RBD mutations has already emerged according to the GISAID database, it is necessary to develop vaccines that protect against such complete breakthrough variants.
17. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion | Nature
Abstract
The SARS-CoV-2 B.1.617.2 (Delta) variant was first identified in the state of Maharashtra in late 2020 and spread throughout India, outcompeting pre-existing lineages including B.1.617.1 (Kappa) and B.1.1.7 (Alpha)1. In vitro, B.1.617.2 is 6-fold less sensitive to serum neutralising antibodies from recovered individuals, and 8-fold less sensitive to vaccine-elicited antibodies as compared to wild type (WT) Wuhan-1 bearing D614G. Serum neutralising titres against B.1.617.2 were lower in ChAdOx-1 versus BNT162b2 vaccinees. B.1.617.2 spike pseudotyped viruses exhibited compromised sensitivity to monoclonal antibodies against the receptor binding domain (RBD) and N- terminal domain (NTD). B.1.617.2 demonstrated higher replication efficiency in both airway organoid and human airway epithelial systems compared to B.1.1.7, associated with B.1.617.2 spike in a predominantly cleaved state compared to B.1.1.7. The B.1.617.2 spike protein was able to mediate highly efficient syncytium formation that was less sensitive to inhibition by neutralising antibody as compared to WT spike. Additionally we observed that B.1.617.2 had higher replication and spike mediated entry as compared to B.1.617.1, potentially explaining B.1.617.2 dominance. In an analysis of over 130 SARS-CoV-2 infected healthcare workers across three centres in India during a period of mixed lineage circulation, we observed reduced ChAdOx-1 vaccine effectiveness against B.1.617.2 relative to non- B.1.617.2, with the caveat of possible residual confounding. Compromised vaccine efficacy against the highly fit and immune evasive B.1.617.2 Delta variant warrants continued infection control measures in the post-vaccination era.
The dynamics of immunity following second doses differed significantly between BNT162b2 and ChAdOx1, with greater initial effectiveness against new PCR-positives but faster declines in protection against high viral burden and symptomatic infection with BNT162b2. There was no evidence that effectiveness varied by dosing interval, but protection was higher among those vaccinated following a prior infection and younger adults. With Delta, infections occurring following two vaccinations had similar peak viral burden to those in unvaccinated individuals. SARS-CoV-2 vaccination still reduces new infections, but effectiveness and attenuation of peak viral burden are reduced with Delta.
At the country-level, there appears to be no discernable relationship between percentage of population fully vaccinated and new COVID-19 cases in the last 7 days (Fig. 1). In fact, the trend line suggests a marginally positive association such that countries with higher percentage of population fully vaccinated have higher COVID-19 cases per 1 million people. Notably, Israel with over 60% of their population fully vaccinated had the highest COVID-19 cases per 1 million people in the last 7 days. The lack of a meaningful association between percentage population fully vaccinated and new COVID-19 cases is further exemplified, for instance, by comparison of Iceland and Portugal. Both countries have over 75% of their population fully vaccinated and have more COVID-19 cases per 1 million people than countries such as Vietnam and South Africa that have around 10% of their population fully vaccinated.
Associations between vaccine breakthrough cases and infection by SARS coronavirus 2 (SARS-CoV-2) variants have remained largely unexplored. Here we analyzed SARS-CoV-2 whole-genome sequences and viral loads from 1,373 persons with COVID-19 from the San Francisco Bay Area from February 1 to June 30, 2021, of which 125 (9.1%) were vaccine breakthrough infections. Fully vaccinated were more likely than unvaccinated persons to be infected by variants carrying mutations associated with decreased antibody neutralization (L452R, L452Q, E484K, and/or F490S) (78% versus 48%, p = 1.96e-08), but not by those associated with increased infectivity (L452R and/or N501Y) (85% versus 77%, p = 0.092). Differences in viral loads were non-significant between unvaccinated and fully vaccinated persons overall (p = 0.99) and according to lineage (p = 0.09 – 0.78). Viral loads were significantly higher in symptomatic as compared to asymptomatic vaccine breakthrough cases (p < 0.0001), and symptomatic vaccine breakthrough infections had similar viral loads to unvaccinated infections (p = 0.64). In 5 cases with available longitudinal samples for serologic analyses, vaccine breakthrough infections were found to be associated with low or undetectable neutralizing antibody levels attributable to immunocompromised state or infection by an antibody-resistant lineage. These findings suggest that vaccine breakthrough cases are preferentially caused by circulating antibody-resistant SARS-CoV-2 variants, and that symptomatic breakthrough infections may potentially transmit COVID-19 as efficiently as unvaccinated infections, regardless of the infecting lineage.
Re Infection/Prior Immunity:
1. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection | Science
Immune memory against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) helps to determine protection against reinfection, disease risk, and vaccine efficacy. Using 188 human cases across the range of severity of COVID-19, Dan et al. analyzed cross-sectional data describing the dynamics of SARS-CoV-2 memory B cells, CD8+ T cells, and CD4+ T cells for more than 6 months after infection. The authors found a high degree of heterogeneity in the magnitude of adaptive immune responses that persisted into the immune memory phase to the virus. However, immune memory in three immunological compartments remained measurable in greater than 90% of subjects for more than 5 months after infection. Despite the heterogeneity of immune responses, these results show that durable immunity against secondary COVID-19 disease is a possibility for most individuals.
Persistent viral infections are commonly accompanied by immunologic dysregulation, especially within the cellular immune compartment. However, it is unclear if persistent mild-to-moderate COVID-19 impacts the development of virus-specific cellular immunity. To this end, we analyzed the development of SARS-CoV-2 specific cellular immunity in convalescent COVID-19 patients who experienced eight days or fewer of COVID-19 symptoms, or symptoms persisting for 18 days or more. We observed that the duration of COVID-19 symptoms minimally impacts the magnitude, antigen specificity, and transcriptional profile of SARS-CoV-2 specific cellular immunity within both the CD4+ and CD8+ T cell compartments. Furthermore, we observed that reactivity against the structural N protein from SARS-CoV-2 in convalescent COVID-19 patients correlates with the amount of reactivity against the seasonal human coronaviruses 229E and NL63. These results provide additional insight into the complex processes that regulate the development of cellular immunity against SARS-CoV-2 and related human coronaviruses.
3. Lasting immunity found after recovery from COVID-19 | National Institutes of Health (NIH)
At a Glance
· The immune systems of more than 95% of people who recovered from COVID-19 had durable memories of the virus up to eight months after infection.
· The results provide hope that people receiving SARS-CoV-2 vaccines will develop similar lasting immune memories after vaccination.
CD8+ T cells are important antiviral effectors that can potentiate long-lived immunity against COVID-19, but a detailed characterization of these cells has been hampered by technical challenges. We screened 21 well-characterized, longitudinally-sampled convalescent donors that recovered from mild COVID-19 against a collection of SARS-CoV-2 tetramers, and identified one participant with an immunodominant response against Nuc322-331, a peptide that is conserved in all the SARS-CoV-2 variants-of-concern reported to date. We conducted 38- parameter CyTOF phenotyping on tetramer-identified Nuc322-331-specific CD8+ T cells, and on CD4+ and CD8+ T cells recognizing the entire nucleocapsid and spike proteins from SARS- CoV-2, and took 32 serological measurements on longitudinal specimens from this participant. We discovered a coordination of the Nuc322-331-specific CD8+ T response with both the CD4+ T cell and antibody pillars of adaptive immunity. Nuc322-331-specific CD8+ T cells were predominantly central memory T cells, but continually evolved over a ∼6-month period of convalescence. We observed a slow and progressive decrease in the activation state and polyfunctionality of the Nuc322-331-specific CD8+ T cells, accompanied by an increase in their lymph-node homing and homeostatic proliferation potential. These results suggest that following a typical case of mild COVID-19, SARS-CoV-2-specific CD8+ T cells not only persist but continuously differentiate in a coordinated fashion well into convalescence, into a state characteristic of long-lived, self-renewing memory.
We performed an immune comparative analysis of SARS-CoV-2 infected individuals stratified by the absence or presence of seroconversion and disease severity. We report high levels of total naïve and low effector CD8+ T cells in NSC. Moreover, polyfunctional Nucleocapsid (NP)-specific CD8+ T-cell responses, as well as reduced levels of T-cell activation monitored by PD-1 and activation-induced markers, were distinctive immunological traits in NSC. Longitudinal data support the stability of the NSC phenotype over three months. Our results implicate highly functional SARS-CoV-2 Spike and NP T-cell responses with low immune activation in protection from disease severity in the absence of seroconversion.
6. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls | Nature
Memory T cells induced by previous pathogens can shape susceptibility to, and the clinical severity of, subsequent infections1. Little is known about the presence in humans of pre-existing memory T cells that have the potential to recognize severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Here we studied T cell responses against the structural (nucleocapsid (N) protein) and non-structural (NSP7 and NSP13 of ORF1) regions of SARS-CoV-2 in individuals convalescing from coronavirus disease 2019 (COVID-19) (n = 36). In all of these individuals, we found CD4 and CD8 T cells that recognized multiple regions of the N protein. Next, we showed that patients (n = 23) who recovered from SARS (the disease associated with SARS-CoV infection) possess long-lasting memory T cells that are reactive to the N protein of SARS-CoV 17 years after the outbreak of SARS in 2003; these T cells displayed robust cross-reactivity to the N protein of SARS-CoV-2. We also detected SARS-CoV-2-specific T cells in individuals with no history of SARS, COVID-19 or contact with individuals who had SARS and/or COVID-19 (n = 37). SARS-CoV-2-specific T cells in uninfected donors exhibited a different pattern of immunodominance, and frequently targeted NSP7 and NSP13 as well as the N protein. Epitope characterization of NSP7-specific T cells showed the recognition of protein fragments that are conserved among animal betacoronaviruses but have low homology to ‘common cold’ human-associated coronaviruses. Thus, infection with betacoronaviruses induces multi-specific and long-lasting T cell immunity against the structural N protein. Understanding how pre-existing N- and ORF1-specific T cells that are present in the general population affect the susceptibility to and pathogenesis of SARS-CoV-2 infection is important for the management of the current COVID-19 pandemic.
7. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers | NEJM
RESULTS
A total of 12,541 health care workers participated and had anti-spike IgG measured; 11,364 were followed up after negative antibody results and 1265 after positive results, including 88 in whom seroconversion occurred during follow-up. A total of 223 anti-spike–seronegative health care workers had a positive PCR test (1.09 per 10,000 days at risk), 100 during screening while they were asymptomatic and 123 while symptomatic, whereas 2 anti-spike–seropositive health care workers had a positive PCR test (0.13 per 10,000 days at risk), and both workers were asymptomatic when tested (adjusted incidence rate ratio, 0.11; 95% confidence interval, 0.03 to 0.44; P=0.002). There were no symptomatic infections in workers with anti-spike antibodies. Rate ratios were similar when the anti-nucleocapsid IgG assay was used alone or in combination with the anti-spike IgG assay to determine baseline status.
8. T cell Epitopes: adaptive immune response against COVID-19.
Over the past year, numerous studies in the peer reviewed and preprint literature have reported on the virological, epidemiological and clinical characteristics of the coronavirus, SARS-CoV-2. To date, 25 studies have investigated and identified SARS-CoV-2-derived T cell epitopes in humans. Here, we review these recent studies, how they were performed, and their findings. We review how epitopes identified throughout the SARS-CoV2 proteome reveal significant correlation between number of epitopes defined and size of the antigen provenance. We also report additional analysis of SARS-CoV-2 human CD4 and CD8 T cell epitope data compiled from these studies, identifying 1,400 different reported SARS-CoV-2 epitopes and revealing discrete immunodominant regions of the virus and epitopes that are more prevalently recognized. This remarkable breadth of epitope repertoire has implications for vaccine design, cross-reactivity, and immune escape by SARS-CoV-2 variants.
9. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans | Nature
Long-lived bone marrow plasma cells (BMPCs) are a persistent and essential source of protective antibodies1,2,3,4,5,6,7. Individuals who have recovered from COVID-19 have a substantially lower risk of reinfection with SARS-CoV-28,9,10. Nonetheless, it has been reported that levels of anti-SARS-CoV-2 serum antibodies decrease rapidly in the first few months after infection, raising concerns that long-lived BMPCs may not be generated and humoral immunity against SARS-CoV-2 may be short-lived11,12,13. Here we show that in convalescent individuals who had experienced mild SARS-CoV-2 infections (n = 77), levels of serum anti-SARS-CoV-2 spike protein (S) antibodies declined rapidly in the first 4 months after infection and then more gradually over the following 7 months, remaining detectable at least 11 months after infection. Anti-S antibody titres correlated with the frequency of S-specific plasma cells in bone marrow aspirates from 18 individuals who had recovered from COVID-19 at 7 to 8 months after infection. S-specific BMPCs were not detected in aspirates from 11 healthy individuals with no history of SARS-CoV-2 infection. We show that S-binding BMPCs are quiescent, which suggests that they are part of a stable compartment. Consistently, circulating resting memory B cells directed against SARS-CoV-2 S were detected in the convalescent individuals. Overall, our results indicate that mild infection with SARS-CoV-2 induces robust antigen-specific, long-lived humoral immune memory in humans.
10. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns | Nature Reviews Immunology
In conclusion, it is now established that SARS-CoV-2 pre-existing immune reactivity exists to some degree in the general population. It is hypothesized, but not yet proven, that this might be due to immunity to CCCs. This might have implications for COVID-19 disease severity, herd immunity and vaccine development, which still await to be addressed with actual data.
Understanding adaptive immunity to SARS-CoV-2 is important for vaccine development, interpreting coronavirus disease 2019 (COVID-19) pathogenesis, and calibration of pandemic control measures. Using HLA class I and II predicted peptide “megapools,” circulating SARS-CoV-2-specific CD8+ and CD4+ T cells were identified in ∼70% and 100% of COVID-19 convalescent patients, respectively. CD4+ T cell responses to spike, the main target of most vaccine efforts, were robust and correlated with the magnitude of the anti-SARS-CoV-2 IgG and IgA titers. The M, spike, and N proteins each accounted for 11%–27% of the total CD4+ response, with additional responses commonly targeting nsp3, nsp4, ORF3a, and ORF8, among others. For CD8+ T cells, spike and M were recognized, with at least eight SARS-CoV-2 ORFs targeted. Importantly, we detected SARS-CoV-2-reactive CD4+ T cells in ∼40%–60% of unexposed individuals, suggesting cross-reactive T cell recognition between circulating “common cold” coronaviruses and SARS-CoV-2.
12. Covid-19: Do many people have pre-existing immunity? | The BMJ
Though these studies are small and do not yet provide precise estimates of pre-existing immunological responses to SARS-CoV-2, they are hard to dismiss, with several being published in Cell and Nature. Alessandro Sette, an immunologist from La Jolla Institute for Immunology in California and an author of several of the studies (box 1), told The BMJ, “At this point there are a number of studies that are seeing this reactivity in different continents, different labs. As a scientist you know that is a hallmark of something that has a very strong footing.”
13. Recent endemic coronavirus infection is associated with less-severe COVID-19 - PubMed
Four different endemic coronaviruses (eCoVs) are etiologic agents for the seasonal common cold, and these eCoVs share extensive sequence homology with human SARS coronavirus 2 (SARS-CoV-2). Here, we show that individuals with, as compared with those without, a recent documented infection with eCoV were tested at greater frequency for respiratory infections but had a similar rate of SARS-CoV-2 acquisition. Importantly, the patients with a previously detected eCoV had less-severe coronavirus disease 2019 (COVID-19) illness. Our observations suggest that preexisting immune responses against endemic human coronaviruses can mitigate disease manifestations from SARS-CoV-2 infection.
In this investigation we examined the magnitude, breadth, and durability of SARS-CoV-2 specific antibodies in two distinct B-cell compartments: long-lived plasma cell-derived antibodies in the plasma, and peripheral memory B-cells along with their associated antibody profiles elicited after in vitro stimulation. We found that magnitude varied amongst individuals, but was the highest in hospitalized subjects. Variants of concern (VoC) -RBD-reactive antibodies were found in the plasma of 72% of samples in this investigation, and VoC-RBD-reactive memory B-cells were found in all but 1 subject at a single time-point. This finding, that VoC-RBD-reactive MBCs are present in the peripheral blood of all subjects including those that experienced asymptomatic or mild disease, provides a reason for optimism regarding the capacity of vaccination, prior infection, and/or both, to limit disease severity and transmission of variants of concern as they continue to arise and circulate.
15. Naturally enhanced neutralizing breadth to SARS-CoV-2 after one year | bioRxiv
Over one year after its inception, the coronavirus disease-2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) remains difficult to control despite the availability of several excellent vaccines. Progress in controlling the pandemic is slowed by the emergence of variants that appear to be more transmissible and more resistant to antibodies1,2. Here we report on a cohort of 63 COVID-19-convalescent individuals assessed at 1.3, 6.2 and 12 months after infection, 41% of whom also received mRNA vaccines3,4. In the absence of vaccination antibody reactivity to the receptor binding domain (RBD) of SARS-CoV-2, neutralizing activity and the number of RBD-specific memory B cells remain relatively stable from 6 to 12 months. Vaccination increases all components of the humoral response, and as expected, results in serum neutralizing activities against variants of concern that are comparable to or greater than neutralizing activity against the original Wuhan Hu-1 achieved by vaccination of naïve individuals2,5-8. The mechanism underlying these broad-based responses involves ongoing antibody somatic mutation, memory B cell clonal turnover, and development of monoclonal antibodies that are exceptionally resistant to SARS-CoV-2 RBD mutations, including those found in variants of concern4,9. In addition, B cell clones expressing broad and potent antibodies are selectively retained in the repertoire over time and expand dramatically after vaccination. The data suggest that immunity in convalescent individuals will be very long lasting and that convalescent individuals who receive available mRNA vaccines will produce antibodies and memory B cells that should be protective against circulating SARS-CoV-2 variants.
Observational data from a national clinical laboratory, though limited by an epidemiological view of the U.S. population, offer an encouraging timeline for the development and sustainability of antibodies up to ten months from natural infection and could inform post-pandemic planning.
17. Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors | Nature
Here we show that of the 32 individuals tested that were born in or before 1915, each showed seroreactivity with the 1918 virus, nearly 90 years after the pandemic. Seven of the eight donor samples tested had circulating B cells that secreted antibodies that bound the 1918 HA. We isolated B cells from subjects and generated five monoclonal antibodies that showed potent neutralizing activity against 1918 virus from three separate donors. These antibodies also cross-reacted with the genetically similar HA of a 1930 swine H1N1 influenza strain, but did not cross-react with HAs of more contemporary human influenza viruses. The antibody genes had an unusually high degree of somatic mutation. The antibodies bound to the 1918 HA protein with high affinity, had exceptional virus-neutralizing potency and protected mice from lethal infection. Isolation of viruses that escaped inhibition suggested that the antibodies recognize classical antigenic sites on the HA surface. Thus, these studies demonstrate that survivors of the 1918 influenza pandemic possess highly functional, virus-neutralizing antibodies to this uniquely virulent virus, and that humans can sustain circulating B memory cells to viruses for many decades after exposure—well into the tenth decade of life.
18. SARS-CoV-2 mRNA vaccines induce a robust germinal centre reaction in humans | Research Square
In these participants, neutralizing titers increased more rapidly after immunization, with GMTs of 4,544.1, 3,584.1, and 1,897.1 against D614G, B.1.1.7, and B.1.351, respectively after primary immunization, and 9,381.2, 9,351.4, and 2,748.6 against D614G, B.1.1.7, and B.1.351 respectively after secondary immunization. These GMTs were 78-, 73-, and 53-fold higher after primary immunization and 16-, 25-, and 20-fold higher after boosting against D614G, B.1.1.7, and B.1.351, respectively than participants without history of SARS-CoV-2 infection.
The results in Fig. 2 show that IgG and IgM antibodies to S2 and N were more common than antibodies to S1 and RBD, reaching 100% with S2 and N for IgG antibodies in the eight sera obtained between days 51 to 65. RBD was least frequently detected among the four antigens used in the IBs, with RBD-reactivity ranging from 0–17% for IgM and 0–25% for IgG antibodies in the different time periods. Of the 37 COVID-19 patients, either IgM or IgG antibodies were found reacting in the COVID-19 IBs with each of the proteins S1, S2, N and RBD in 23, 35, 33 and 7 patients respectively.
Virus-specific humoral and cellular immunity act synergistically to protect the host from viral infection. We interrogate the dynamic changes of virological and immunological parameters in 12 patients with symptomatic acute SARS-CoV-2 infection from disease onset to convalescence or death. We quantify SARS-CoV-2 viral RNA in the respiratory tract in parallel with antibodies and circulating T cells specific for various structural (nucleoprotein [NP], membrane [M], ORF3a, and spike) and non-structural (ORF7/8, NSP7, and NSP13) proteins. Although rapid induction and quantity of humoral responses associate with an increase in disease severity, early induction of interferon (IFN)-γ-secreting SARS-CoV-2-specific T cells is present in patients with mild disease and accelerated viral clearance. These findings provide support for the prognostic value of early functional SARS-CoV-2-specific T cells with important implications in vaccine design and immune monitoring.
Previously, it has been shown that antibody levels wane with time in SARS-CoV-1 infection, while cellular immunity can last 6 to 11 years (8–13). Similarly, a recent study of antibody levels revealed that 40% of asymptomatic and 13% of symptomatic patients infected by SARS-CoV-2 became negative for immunoglobulin G eight weeks post-recovery (14). Exposing T cells from recovered SARS-CoV-1 patients to peptides derived from the S protein of this virus revealed that the induction of polyfunctional T cells (T cells producing multiple effector cytokines) was higher in individuals with severe SARS-CoV-1 infection than in those with moderate infection, indicating that the level of T-cell response corresponds with the severity of this infection and time to recovery (15). A recent study in recovered COVID-19 patients revealed that even in the absence of antibodies to SARS-CoV-2, a robust T-cell immune response was measured, indicating the importance of T-cell immunity in response to COVID-19 (16). In particular, T-cell activation/exhaustion and lymphopenia were associated with severe disease, whereas traditional effector functions of CD8+ T cells were related to a better prognosis (17). Since the cellular responses during COVID-19 are complex, longitudinal assessment of both CD4+ and CD8+ T-cell responses can inform how SARS-CoV-2 infection and vaccines for this disease modulate immune functions over time.
T-cell immunity is important for recovery from COVID-19 and provides heightened immunity for re-infection. However, little is known about the SARS-CoV-2-specific T-cell immunity in virus-exposed individuals. Here we report virus-specific CD4+ and CD8+ T-cell memory in recovered COVID-19 patients and close contacts. We also demonstrate the size and quality of the memory T-cell pool of COVID-19 patients are larger and better than those of close contacts. However, the proliferation capacity, size and quality of T-cell responses in close contacts are readily distinguishable from healthy donors, suggesting close contacts are able to gain T-cell immunity against SARS-CoV-2 despite lacking a detectable infection. Additionally, asymptomatic and symptomatic COVID-19 patients contain similar levels of SARS-CoV-2-specific T-cell memory. Overall, this study demonstrates the versatility and potential of memory T cells from COVID-19 patients and close contacts, which may be important for host protection.
This study examined whether CD8+ T-cell responses from coronavirus disease 2019 convalescent individuals (n = 30) potentially maintain recognition of the major severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants (alpha, beta, gamma; n = 45 mutations assessed). Only 1 mutation found in Beta variant-spike overlapped with a previously identified epitope (1/52), suggesting that virtually all anti-SARS-CoV-2 CD8+ T-cell responses should recognize these newly described variants.
24. T cells recognize recent SARS-CoV-2 variants | National Institutes of Health (NIH)
In their study of recovered COVID-19 patients, the researchers determined that SARS-CoV-2-specific CD8+ T-cell responses remained largely intact and could recognize virtually all mutations in the variants studied. While larger studies are needed, the researchers note that their findings suggest that the T cell response in convalescent individuals, and most likely in vaccinees, are largely not affected by the mutations found in these three variants, and should offer protection against emerging variants.
Results
In 14/33 (42.4%) unexposed donors and 85/87 (97.7%) COVID-19 convalescent subjects a positive result for at least one SARS-CoV-2 antigen was observed. A positive response was observed up to 12 months after COVID-19 infection (median 246 days after symptom onset; range 118–362 days). Of note, SARS-CoV-2 T-cell response seems to be mainly mediated by CD4 T cells. A weak positive correlation was observed between Spike-specific T-cell response and neutralizing antibody titre (p 0.0028; r2 = 0.2891) and positive SARS-CoV-2 T-cell response was observed in 8/9 (88.9%) COVID-19 convalescent subjects with undetectable SARS-CoV-2 neutralizing antibodies.
Discussion
Cross-reactive SARS-CoV-2 T-cell response in uninfected patients may be due to previous infections with other common coronaviruses. Our data suggest that long-term SARS-CoV-2 T-cell response might accompany a waning humoral response.
26. A majority of uninfected adults show preexisting antibody reactivity against SARS-CoV-2 - PubMed
Preexisting cross-reactivity to SARS-CoV-2 occurs in the absence of prior viral exposure. However, this has been difficult to quantify at the population level due to a lack of reliably defined seroreactivity thresholds. Using an orthogonal antibody testing approach, we estimated that about 0.6% of nontriaged adults from the greater Vancouver, Canada, area between May 17 and June 19, 2020, showed clear evidence of a prior SARS-CoV-2 infection, after adjusting for false-positive and false-negative test results. Using a highly sensitive multiplex assay and positive/negative thresholds established in infants in whom maternal antibodies have waned, we determined that more than 90% of uninfected adults showed antibody reactivity against the spike protein, receptor-binding domain (RBD), N-terminal domain (NTD), or the nucleocapsid (N) protein from SARS-CoV-2. This seroreactivity was evenly distributed across age and sex, correlated with circulating coronaviruses' reactivity, and was partially outcompeted by soluble circulating coronaviruses' spike. Using a custom SARS-CoV-2 peptide mapping array, we found that this antibody reactivity broadly mapped to spike and to conserved nonstructural viral proteins. We conclude that most adults display preexisting antibody cross-reactivity against SARS-CoV-2, which further supports investigation of how this may impact the clinical severity of COVID-19 or SARS-CoV-2 vaccine responses.
In this descriptive preliminary report, we conducted a large-scale assessment on the country level of the possible occurrence of COVID-19 reinfection within the members of a large healthcare provider in Israel. Out of 149,735 individuals with a documented positive PCR test between March 2020 and January 2021, 154 had two positive PCR tests at least 100 days apart, reflecting a reinfection proportion of 1 per 1000. Given our strict inclusion criteria, we believe these numbers represent true reinfection incidence in MHS and should be clinically regarded as such.
The efficacy of virus-specific T cells in clearing pathogens involves a fine balance between antiviral and inflammatory features. SARS-CoV-2–specific T cells in individuals who clear SARS-CoV-2 without symptoms could reveal nonpathological yet protective characteristics. We longitudinally studied SARS-CoV-2–specific T cells in a cohort of asymptomatic (n = 85) and symptomatic (n = 75) COVID-19 patients after seroconversion. We quantified T cells reactive to structural proteins (M, NP, and Spike) using ELISpot and cytokine secretion in whole blood. Frequencies of SARS-CoV-2–specific T cells were similar between asymptomatic and symptomatic individuals, but the former showed an increased IFN-γ and IL-2 production. This was associated with a proportional secretion of IL-10 and proinflammatory cytokines (IL-6, TNF-α, and IL-1β) only in asymptomatic infection, while a disproportionate secretion of inflammatory cytokines was triggered by SARS-CoV-2–specific T cell activation in symptomatic individuals. Thus, asymptomatic SARS-CoV-2–infected individuals are not characterized by weak antiviral immunity; on the contrary, they mount a highly functional virus-specific cellular immune response.
29. Quantifying the risk of SARS‐CoV‐2 reinfection over time
A standardised protocol was employed, based on Cochrane methodology. Electronic databases and preprint servers were searched from 1 January 2020 to 19 February 2021. Eleven large cohort studies were identified that estimated the risk of SARS‐CoV‐2 reinfection over time, including three that enrolled healthcare workers and two that enrolled residents and staff of elderly care homes. Across studies, the total number of PCR‐positive or antibody‐positive participants at baseline was 615,777, and the maximum duration of follow‐up was more than 10 months in three studies. Reinfection was an uncommon event (absolute rate 0%–1.1%), with no study reporting an increase in the risk of reinfection over time. Only one study estimated the population‐level risk of reinfection based on whole genome sequencing in a subset of patients; the estimated risk was low (0.1% [95% CI: 0.08–0.11%]) with no evidence of waning immunity for up to 7 months following primary infection. These data suggest that naturally acquired SARS‐CoV‐2 immunity does not wane for at least 10 months post‐infection.
Reinfection is rare in the young and international population of Qatar. Natural infection appears to elicit strong protection against reinfection with an efficacy ~95% for at least seven months.
The study sought to examine the rate of reinfection following initial infection using a retrospective cohort in Ohio and Florida from March 20, 2020 to February 24, 2021. The study found that, of 150,325 people tested during this period in the health system before August 30, 2020, 1,278 patients who initially tested positive later were tested again, 63 (4.9%) showed evidence of reinfection. Protection from reinfection due to prior infection was estimated at 81.8%. The study’s primary limitation was the use of infection occurrence within 6-month time blocks to infer re-infection, rather than having an initial positive test, a subsequent negative test, and a later positive test to determine that individuals cleared the infection.
During the first surge (ie, before June, 2020), 533 381 people were tested, of whom 11 727 (2·20%) were PCR positive, and 525 339 were eligible for follow-up in the second surge, of whom 11 068 (2·11%) had tested positive during the first surge. Among eligible PCR-positive individuals from the first surge of the epidemic, 72 (0·65% [95% CI 0·51–0·82]) tested positive again during the second surge compared with 16 819 (3·27% [3·22–3·32]) of 514 271 who tested negative during the first surge (adjusted RR 0·195 [95% CI 0·155–0·246]). Protection against repeat infection was 80·5% (95% CI 75·4–84·5). The alternative cohort analysis gave similar estimates (adjusted RR 0·212 [0·179–0·251], estimated protection 78·8% [74·9–82·1]). In the alternative cohort analysis, among those aged 65 years and older, observed protection against repeat infection was 47·1% (95% CI 24·7–62·8). We found no difference in estimated protection against repeat infection by sex (male 78·4% [72·1–83·2] vs female 79·1% [73·9–83·3]) or evidence of waning protection over time (3–6 months of follow-up 79·3% [74·4–83·3] vs ≥7 months of follow-up 77·7% [70·9–82·9]).
In this study, we have found that a first infection with the A/Brisbane/59/2007 virus strain confers heterologous protection in ferrets and mice against a subsequent pdmH1N1 (A/Mexico/4108/2009) virus infection through a cross-reactive but non-neutralizing antibody mechanism. Heterologous immunity is abrogated in B cell-deficient mice but maintained in CD8−/− and perforin-1−/− mice. We identified cross-reactive antibodies from A/Brisbane/59/2007 sera that recognize non-HA epitopes in pdmH1N1 virus. Passive serum transfer showed that cross-reactive sH1N1-induced antibodies conferred protection in naive recipient mice during pdmH1N1 virus challenge. The presence or absence of anti-HA antibodies, therefore, is not the sole indicator of the effectiveness of protective cross-reactive antibody immunity. Measurement of additional antibody repertoires targeting the non-HA antigens of influenza virus should be taken into consideration in assessing protection and immunization strategies. We propose that preexisting cross-protective non-HA antibody immunity may have had an overall protective effect during the 2009 pdmH1N1 outbreak, thereby reducing disease severity in human infections.
34.
35. Presence of SARS-CoV-2-reactive T cells in COVID-19 patients and healthy donors | medRxiv
Strikingly, S-reactive CD4+ T cells in COVID-19 patients equally targeted N-terminal and C-terminal epitopes of S whereas in HD S-reactive CD4+ T cells reacted almost exclusively to the C-terminal epitopes that are a) characterized by higher homology with spike glycoprotein of human endemic “common cold” coronaviruses (hCoVs), and b) contains the S2 subunit of S with the cytoplasmic peptide (CP), the fusion peptide (FP), and the transmembrane domain (TM) but not the receptor-binding domain (RBD). In contrast to S-reactive CD4+ T cells in HD, S-reactive CD4+ T cells from COVID-19 patients co-expressed CD38 and HLA-DR, indivative of their recent in vivo activation. Our study is the first to directly measure SARS-CoV-2-reactive T cell responses providing critical tools for large scale testing and characterization of potential cross-reactive cellular immunity to SARS-CoV-2. The presence of pre-existing SARS-CoV-2-reactive T cells in a subset of SARS-CoV-2 naïve HD is of high interest but larger scale prospective cohort studies are needed to assess whether their presence is a correlate of protection or pathology for COVID-19.
36. The new SARS-CoV-2 variant and reinfection in the resurgence of COVID-19 outbreaks in Manaus, Brazil
Manaus, a city of 2.2 million population, the capital of Amazonas state of Brazil was hit badly by two waves of COVID-19 with more than 10,000 severe acute respiratory syndrome deaths by the end of February 2021. It was estimated that the first wave infected over three quarters of the population in Manaus based on routine blood donor data, and the second wave was largely due to reinfection with a new variant named P1 strain.
37. Persistence of neutralizing antibodies a year after SARS-CoV-2 infection
We found that NAb against the wild-type virus and S-IgG persisted in 89% and 97% of subjects for at least twelve months after infection, respectively. IgG and NAb levels were higher after severe infection. NAb titers were significantly lower against variants compared to the wild-type virus.
Developing effective strategies to prevent or treat coronavirus disease 2019 (COVID-19) requires understanding the natural immune response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We used an unbiased, genome-wide screening technology to determine the precise peptide sequences in SARS-CoV-2 that are recognized by the memory CD8+ T cells of COVID-19 patients. In total, we identified 3–8 epitopes for each of the 6 most prevalent human leukocyte antigen (HLA) types. These epitopes were broadly shared across patients and located in regions of the virus that are not subject to mutational variation. Notably, only 3 of the 29 shared epitopes were located in the spike protein, whereas most epitopes were located in ORF1ab or the nucleocapsid protein. We also found that CD8+ T cells generally do not cross-react with epitopes in the four seasonal coronaviruses that cause the common cold. Overall, these findings can inform development of next-generation vaccines that better recapitulate natural CD8+ T cell immunity to SARS-CoV-2.
Highlights
Most recovered COVID-19 patients mount broad, durable immunity after infection
Neutralizing antibodies show a bi-phasic decay with half-lives >200 days
Spike IgG+ memory B cells increase and persist post-infection
Durable polyfunctional CD4 and CD8 T cells recognize distinct viral epitope regions
Summary
Ending the COVID-19 pandemic will require long-lived immunity to SARS-CoV-2. Here, we evaluate 254 COVID-19 patients longitudinally up to 8 months and find durable broad-based immune responses. SARS-CoV-2 spike binding and neutralizing antibodies exhibit a bi-phasic decay with an extended half-life of >200 days suggesting the generation of longer-lived plasma cells. SARS-CoV-2 infection also boosts antibody titers to SARS-CoV-1 and common betacoronaviruses. In addition, spike-specific IgG+ memory B cells persist, which bodes well for a rapid antibody response upon virus re-exposure or vaccination. Virus-specific CD4+ and CD8+ T cells are polyfunctional and maintained with an estimated half-life of 200 days. Interestingly, CD4+ T cell responses equally target several SARS-CoV-2 proteins, whereas the CD8+ T cell responses preferentially target the nucleoprotein, highlighting the potential importance of including the nucleoprotein in future vaccines. Taken together, these results suggest that broad and effective immunity may persist long-term in recovered COVID-19 patients.
This article proposes that there is a significant role for mucosal immunity and for secretory as well as circulating IgA antibodies in COVID-19, and that it is important to elucidate this in order to comprehend especially the asymptomatic and mild states of the infection, which appear to account for the majority of cases. Moreover, it is possible that mucosal immunity can be exploited for beneficial diagnostic, therapeutic, or prophylactic purposes.
41. Had COVID? You’ll probably make antibodies for a lifetime
People who recover from mild COVID-19 have bone-marrow cells that can churn out antibodies for decades.
42. Immunity after SARS-CoV-2 infections | Nature Immunology
A central question related to SARS-CoV-2 immunity is whether infection establishes a reservoir of memory cells against this pathogen that are capable of fending off a subsequent infection. This study is reassuring, as the majority of individuals who were infected six months earlier, even if they experienced no symptoms or mild symptoms during infection, were capable of mounting a cellular immune response against this pathogen.
Memory T cells contribute to rapid viral clearance during re-infection, but the longevity and differentiation of SARS-CoV-2-specific memory T cells remain unclear. Here we conduct ex vivo assays to evaluate SARS-CoV-2-specific CD4+ and CD8+ T cell responses in COVID-19 convalescent patients up to 317 days post-symptom onset (DPSO), and find that memory T cell responses are maintained during the study period regardless of the severity of COVID-19. In particular, we observe sustained polyfunctionality and proliferation capacity of SARS-CoV-2-specific T cells. Among SARS-CoV-2-specific CD4+ and CD8+ T cells detected by activation-induced markers, the proportion of stem cell-like memory T (TSCM) cells is increased, peaking at approximately 120 DPSO. Development of TSCM cells is confirmed by SARS-CoV-2-specific MHC-I multimer staining. Considering the self-renewal capacity and multipotency of TSCM cells, our data suggest that SARS-CoV-2-specific T cells are long-lasting after recovery from COVID-19, thus support the feasibility of effective vaccination programs as a measure for COVID-19 control.
44. Durability of SARS-CoV-2-specific T cell responses at 12-months post-infection | bioRxiv
Results SARS-CoV-2-specific antibodies and T cells were detected at 12-months post-infection. Severity of acute illness was associated with higher frequencies of SARS-CoV-2-specific CD4 T cells and antibodies at 12-months. In contrast, polyfunctional and cytotoxic T cells responsive to SARS-CoV-2 were identified in participants over a wide spectrum of disease severity.
Conclusions Our data show that SARS-CoV-2 infection induces polyfunctional memory T cells detectable at 12-months post-infection, with higher frequency noted in those who originally experienced severe disease.
Although the protective role of antibodies is currently unknown, our results show that SARS-CoV-2 antibodies persisted at least 12 months after symptom onset and maybe even longer, indicating that COVID-19-convalescent individuals may be protected from reinfection. Our results represent SARS-CoV-2 antibody immunity in nationwide cohorts in a setting with few undetected cases [7], and we believe that our results add to the understanding of natural immunity and the expected durability of SARS-CoV-2 vaccine immune responses.
Children have reduced severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates and a substantially lower risk for developing severe coronavirus disease 2019 compared with adults. However, the molecular mechanisms underlying protection in younger age groups remain unknown. Here we characterize the single-cell transcriptional landscape in the upper airways of SARS-CoV-2-negative (n = 18) and age-matched SARS-CoV-2-positive (n = 24) children and corresponding samples from adults (n = 44), covering an age range of 4 weeks to 77 years. Children displayed higher basal expression of relevant pattern recognition receptors such as MDA5 (IFIH1) and RIG-I (DDX58) in upper airway epithelial cells, macrophages and dendritic cells, resulting in stronger innate antiviral responses upon SARS-CoV-2 infection than in adults. We further detected distinct immune cell subpopulations including KLRC1 (NKG2A)+ cytotoxic T cells and a CD8+ T cell population with a memory phenotype occurring predominantly in children. Our study provides evidence that the airway immune cells of children are primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults.
Results Overall, 33.76% of SARS-CoV-2 exposed children and 57.88% adults were seropositive. Children were five times more likely to have seroconverted without symptoms compared to adults. Despite the frequently asymptomatic course of infection, children had higher specific antibody levels, and their antibodies persisted longer than in adults (96.22% versus 82.89% still seropositive 11-12 months post infection). Of note, symptomatic and asymptomatic infections induced similar humoral responses in all age groups. In symptomatic children, only dysgeusia was found as diagnostic indicator of COVID-19. SARS-CoV-2 infections occurred independent of HCoV serostatus. Antibody binding responses to VOCs were similar in children and adults, with reduced binding for the Beta variant in both groups.
Conclusions The long-term humoral immune response to SARS-CoV-2 infection in children is robust and may provide long-term protection even after asymptomatic infection.
Kids armed with anti-coronavirus B cells
It remains unclear whether B cell repertoires against coronaviruses and other pathogens differ between adults and children and how important these distinctions are. Yang et al. analyzed blood samples from young children and adults, as well as tissues from deceased organ donors, characterizing the B cell receptor (BCR) repertoires specific to six common pathogens and two viruses that they had not seen before: Ebola virus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Children had higher frequencies of B cells with convergent BCR heavy chains against previously encountered pathogens and higher frequencies of class-switched convergent B cell clones against SARS-CoV-2 and related coronaviruses. These findings suggest that encounters with coronaviruses in early life may produce cross-reactive memory B cell populations that contribute to divergent COVID-19 susceptibilities.
Methods
This retrospective cohort study included all the population of an Italian Province, diagnosed with a SARS-CoV-2 infection from March 2020 to May 2021. The primary outcome was the incidence of a reinfection, defined as a new positive polymerase chain reaction (PCR) test occurring ≥90 days after complete resolution of the first infection, and data were retrieved from the official datasets (coronavirus disease 2019 [COVID-19], demographic, hospital and co-pay exemption) of the Local Health Unit (LHU) of Pescara.
Results
After an average of 201 days of follow-up (max. 414), we recorded 24 reinfections ≥90 days after the resolution of the first 7173 infections (0.33%). Four reinfections required hospitalization, one was lethal. Most of the reinfections (n = 13) occurred 6–9 months after the resolution of the first infection; no new infection was detected 12 or more months later and among the 832 minors.
Conclusions
This study confirms previous findings on a low risk of SARS-CoV-2 reinfection. If confirmed, these findings suggest that more targeted restriction policies can be applied to the subjects that recovered after a first infection.
Just for kicks: