Dr. Byram Bridle on the Misuse of PCR Testing for Covid
A technically accurate explanation of how PCR tests work and how they can be abused and misused
Dr. Byram Bridle is one of the world’s pre-eminent experts on all things covid. He is one of the few genuine scientists who actually has the relevant qualifications and subject matter expertise to be properly literate:
https://viralimmunologist.substack.com/about
Who is Dr. Byram Bridle?
I am an Associate Professor of Viral Immunology in the Department of Pathobiology at the University of Guelph. I specialize in vaccinology and am also leader of the Vaccine Task Force of the Canadian COVID Care Alliance’s Scientific and Medical Advisory Committee.
My research program focuses on the development of vaccines to prevent infectious diseases and treat cancers, as well as studying the body’s immune response to viruses. I teach several courses at the undergraduate and graduate levels on the topics of immunology, virology, and cancer biology. The overall aim of my research efforts is to develop safe and effective new immunotherapies for people. Indeed, one of my previous cancer vaccine strategies progressed into four human clinical trials.
I am also involved in training Canada’s next generation of multidisciplinary researchers, especially in vaccinology. I received funding from the Ontario Government (COVID-19 Rapid Research Fund, Ministry of Colleges and Universities) and the Government of Canada (Pandemic Response Challenge Program, National Research Council of Canada) to conduct pre-clinical research with vaccines against COVID-19. I also hold numerous grants in support of my cancer research and basic viral immunology research programs, including but not limited to the Canadian Institutes for Health Research, Natural Sciences and Engineering Research Council of Canada, Terry Fox Research Institute, Canadian Cancer Society, and Cancer Research Society. I have received numerous teaching and research awards including the prestigious Terry Fox Research Institute’s New Investigator Award and the Zoetis Award for Research Excellence.
I have served as an expert witness for court cases related to the science of COVID-19, including vaccines.
Since the COVID-19 pandemic was declared, I have been actively involved in providing fact-based, balanced, scientific answers to questions posed by the public to help them make fully informed decisions. This has included ~300 media engagements ranging from radio shows, published articles, and appearances on televised news programs, spanning the local to international scope. I have also been an invited keynote speaker at international conferences that focused on COVID-19 and have served as an invited member of numerous COVID-19-focused discussion panels.
One of his substack posts has what I consider to be one of the most comprehensive and systematic refutations of the entirety of the Public Health response and narrative to date.
The background here is that Dr. Bridle is serving as an expert witness in a court case in Canada regarding the vaccine mandates there. He submitted a massive affidavit to the court that unequivocally demolishes the government’s covid response from top to bottom. It is a shame that his affidavit has basically zero publicity as far as I can tell. Therefore, I am reproducing sections of it with Dr. Bridle’s permission on my substack (with a bit of ‘translation’ from the technical academic vernacular to make it more readily readable to non-academic laypeople).
Explaining why PCR testing was so wildly inaccurate
Here is what Dr. Byram Bridle wrote on the use of PCR tests for covid case detection, broken down into sections and with some interspersed explanation of what he is saying in less academic language:
Introduction/Background:
5. PCR Testing Should Not Have Been the Gold Standard to Detect SARSCoV-2
A common way to detect the presence of a virus in a clinical sample is to use what is called a nucleic acid test (NAT). These kinds of tests work by detecting the presence of the genetic material (i.e, genome) of the virus. Indeed, viral genomes are composed of building blocks known as nucleic acids. Commonly used NATs fall under the umbrella term ‘nucleic acid amplification tests’ (NAATs). These tests incorporate a step that amplifies or increases the amount of the virus-derived genetic material, thereby making it easier to detect. There are different kinds of NAATs, including but not limited to ‘reverse transcription - polymerase chain reaction’ (RT-PCR), ‘transcription-mediated amplification’ (TMA), and ‘loop-mediated isothermal amplification’ (LAMP). However, since RT-PCR is the most common method being used in laboratory-based testing during the pandemic, that will be the focus of this discussion.
A specific form of PCR is most prevalent for detecting SARS-CoV-2. It is known as ‘real-time RT-PCR’. A real-time PCR is also known as a quantitative PCR and it monitors the amplification of a targeted piece of genetic material. Importantly, it can, in theory, provide information about the relative amount of virus-derived genetic material that was present in a sample (i.e, few versus many viral particles).
A PCR test is designed to detect genetic material made of deoxyribonucleic acid (DNA). However, the genome of SARS-CoV-2 is made of ribonucleic acid (RNA). As such, the PCR test cannot be performed until a reverse transcription step is performed, which copies the genetic code of the viral RNA into DNA, which is much more stable than RNA.
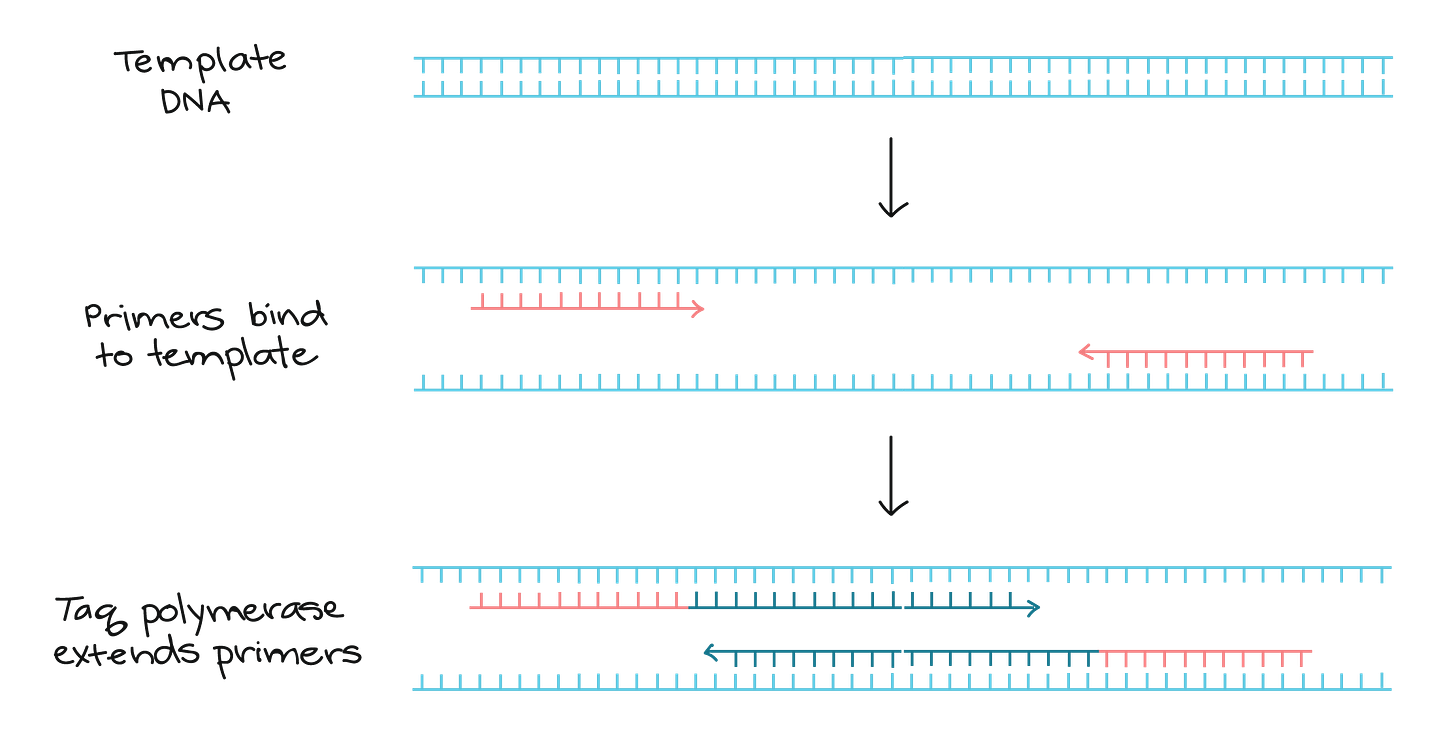
The PCR can then be performed, which involves using what are called ‘primers’ that are designed to bind to unique sequences that are present in a viral genome. The primers are short pieces of DNA that are designed to bind at either end of a segment of the viral genome. If the primers bind, a molecule known as a ‘polymerase’ will use the viral genome as a template to extend the primers until the target gene segment has been completely copied.
In other words, the ‘primer’ piece will bind to the beginning of the section of the genome that the test is designed to look for. If/when it binds, a different type of ‘piece’ will bind to the rest of the chunk of DNA like this (sort of):
This works by varying the temperature of the sample. A high temperature is used to get double-stranded DNA to separate into single strands. Next, an ‘annealing’ temperature is used to allow the primers to bind to the single strands of DNA. Finally, a third temperature is used to promote ‘extension’ of the primers until the targeted gene sequence has been copied. This constitutes a single cycle of the test. Multiple cycles are employed to increase the copies of the targeted gene segment exponentially.
A fluorescent dye is usually added to the sample that incorporates into the targeted gene segment. If enough gene segments get amplified, a special machine can detect the amount of the fluorescent dye. The amount of dye usually correlates with the number of viral genomes in the clinical specimen.
In very layperson language: There is a piece that ‘glows’ that is triggered when the test pieces bind to the entire part of the DNA strand the test is looking for. If enough test pieces bind with DNA strands so that the brightness of these glowing pieces reaches a predetermined threshold, it is a positive test result.
An important piece of information derived from the RT-PCR test is the ‘cycle threshold’ (Ct) value. The Ct value is the number of cycles that the test had to be run for the fluorescent signal to exceed background levels.
There are many steps involved in the optimization of RT-PCR tests before they can be used. If properly designed, a good-quality PCR test can be sensitive enough to detect very small quantities of viral genetic material.
How PCR tests were misused for covid testing
However, when it comes to RT-PCR testing for SARS-CoV2, caution must be exercised when interpreting results. Importantly, poorly optimized RT-PCR tests can have high background signals.
Further, the greater the number of cycles used in a RT-PCR assay, the greater the chance of erroneous non-specific amplification of non-targeted genetic material.
Primer pieces are not perfect, and can sometimes bind with the ‘wrong’ DNA that isn’t the part of the covid genome the test is looking for. When a piece ‘misbinds’, it will however be amplified in each subsequent cycle though, so if there is enough misbinding at the beginning, then running enough cycles can amplify these misbinded pieces into a positive test result.
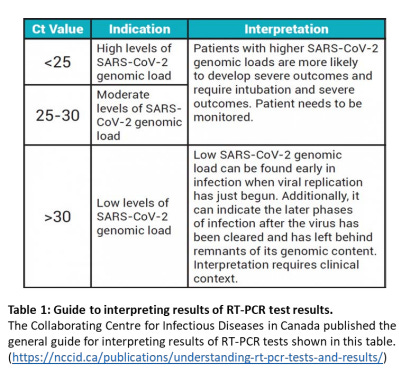
The National Collaborating Centre for Infectious Diseases in Canada published the general guide for interpreting results of RT-PCR tests for SARS-CoV-2 shown in table 1:
In addition to the potential for false signals at high Ct values, note that high values can also be indicative of detection of non-viable viral particles. It is important to note that SARS-CoV-2 particles can exist in two basic forms:
1. Replication-competent; this is the form with the potential to cause COVID-19.
2. Replication incompetent; this cannot cause COVID-19.
Following clearance of SARS-CoV-2 from the body, full and/or partial genomes of SARS-CoV-2 can remain for many days. One key reason for this is that some phagocytic cells, which are a component of the innate immune system, can be long-lived.
The three primary phagocytic cells in the body are neutrophils, macrophages, and dendritic cells.
Neutrophils are the ‘first responders’ of the immune system. They rapidly infiltrate sites of SARS-CoV-2 infection and begin to phagocytose (i.e, consume or internalize) SARS-Cov2 particles. The neutrophils, which are short-lived, then recruit macrophages and dendritic cells to the site of infection.
Note that dendritic cells also reside at strategic sites of infection where they can immediately begin to phagocytose SRAS-CoV-2. The macrophages and dendritic cells are much larger than neutrophils and can phagocytose relatively large quantities of the virus and can be relatively long-lived.
One of the reasons for this is because these two cell types are critical for activating T cells and B cells, which are the key effectors against viral infections. Phagocytosis of SARS-CoV-2 is a mechanism to kill and remove the virus from the body and to activate other immunological effector cells. As such, these can be a source of SARS-CoV-2 genomes that could be amplified by a RT-PCR test.
However, these genomes would not have the potential to cause COVID-19. Persistence of whole or partial genomes that are not associated with infectious particles is well-documented for a variety of viruses, including measles, Middle East respiratory syndrome-coronavirus, and other coronaviruses.
Basically, ‘dead’ virus can be the source for a lot of covid RNA floating around. A PCR test detects the presence of the covid RNA, but can’t distinguish between RNA from a live virus and RNA from a dead virus.
A very recent scientifically peer-reviewed article argued that a reasonable cut-off for cycle numbers for good-quality RT-PCR tests for SARS-CoV-2 is thirty-four. However, most RT-PCR tests for SARS-CoV-2 exceed 34 cycles. For example, Public Health Ontario runs the test at 40 cycles. Their definition of a negative result is if there was no fluorescent signal detected at the end of the full 40 cycles. Any signal detected at the end of 38 cycles is declared to be a positive case. Remarkably, if they detect the viral genome between 38 and 40 cycles, they define the result as a ‘probable case’ for public health reporting.
Jonathan Gubbay, a medical microbiologist with Public Health Ontario, has been quoted on their website as saying the following: "In Ontario, we use PCR as the gold standard of testing for COVID-19 because it is able to successfully detect tiny amounts of the virus (sensitivity) with a low chance for error (accuracy) compared to other types of lab tests.".
The problem is that PCR tests do not represent gold standard assays for determining if potentially infectious viruses are present. Instead, the gold standard assay for this is the inoculation of cultured cell lines and then looking for evidence of infection (e.g. cytopathic effect, which means killing of cells. An in vitro biological assay like this can then be used to correlate Ct values with infectivity of SARS-CoV-2. However, this type of gold standard functional test has not actually been standardized to date in Canada.
If you want to know if there is live virus, the best way to find out is to culture a sample and see if any virus is reproducing. But of course that was not done for covid.
Interpreting the RT-PCR test is challenging, to say the least, without a functional test to compare it to. Of particular concern in the context of the high cycle numbers being used by labs such as those at Public Health Ontario (i.e, 40 cycles, with 38 being defined as ‘positive’), is the fact that several studies have been conducted to determine the highest Ct value at which SARSCoV-2 could be successfully cultured in cells. The results were 25 / 26 / 22-27 / 30 / and 24 for a study conducted at Canada’s own National Microbiology Laboratory. This suggests that tests with CT values above 22-30 are almost certainly not indicative of the presence of replication-competent SARS-CoV-2.
The conclusion is that it is erroneous to declare samples with high Ct values, especially those above 30, as being positive for infectious SARS-CoV-2. It was even concluded in a study by La Scola B, et al. that patients testing ‘positive’ with Ct values above 33-34 could likely be discharged from hospitals. This means that a very large but unknown number of positive cases reported in Ontario were likely not true positives.
RT-PCR-based testing in Ontario is not standardized. Across the province labs use different sample preparation methods, protocols, and gene targets. Variability in CT values (up to 8 cycles). This has prompted Public Health Ontario to discourage the reporting of Ct values <35 alongside test results. Indeed, Ct values <35 are only available upon special request.
The types of specimens and the quality of their collection can influence the results of RTPCR tests. Public Health Ontario recommends this for sample collection for use with the RT-PCR assay: “The gold standard for sample collection method is the nasopharyngeal swab, a swab inserted deep into a person’s nose. However, other sample types exist including combinations of a nose and throat swab and also saliva samples.”
This is of concern because the United States Centres for Disease Control and Prevention “does not recommend NAATs that use oral specimens (e.g., saliva) for confirmatory testing and instead suggests the use of specimens that are considered optimal for detection, such as nasopharyngeal, nasal mid-turbinate, and anterior nasal swabs.”
It is important to note that the problems associated with laboratory-based RT-PCR assays for the detection of SARS-Cov-2 are likely worse for point-of-care tests that rely on similar technology. Indeed, the United States Centres for Disease Control and Prevention acknowledge that “Sensitivity varies by test, but laboratory-based NAATs generally have higher sensitivity than point-of-care tests or tests that can be used anywhere.”.
Further, the United States Centres for Disease Control and Prevention and the United States Food and Drug Administration note the following limitations of RT-PCR tests for SRS-CoV-2:
1. The presence of viral RNA in the sample might not indicate the presence of infectious virus
2. The presence of viral RNA does not necessarily imply that SARS-CoV-2 is the causative agent of COVID-19
3. The test cannot rule out diseases caused by other bacterial or viral pathogens
4. The test is not suitable for screening blood and blood products for the presence of SARS-CoV-2
5. If the virus mutates in the predetermined target region, the test is invalid.
This doesn’t mean that the PCR accuracy is negligible or that it’s fake:
See above, PCR can pick up RNA from dead virus.
This is irrelevant. What it means is that you can’t use the presence of a virus to by itself prove that the virus in question is responsible for a clinical disease - all it shows by itself is correlation, and we all know that correlation doesn’t automatically = causation. However, there is no rational basis to doubt that the covid virus (SARS-CoV-2) causes covid-19 disease. Just ask the doctors treating covid for two years.
This is because it is possible to be sick from, say, RSV while carrying covid virus that is by itself asymptomatic. Or you can be symptomatic from multiple pathogens simultaneously.
I don’t know enough about this offhand to comment on it specifically.
Mutations will lower the binding affinity with the selected portion of the RNA being tested for, raising the rate of false negatives.
Conclusion: RT-PCR tests are based on a remarkable technology. However, they never should have been used as a stand-alone gold standard test for defining cases of COVID-19.
Every lab running RT-PCR tests for the detection of SARS-CoV-2 should have determined an appropriate CT cut-off through parallel testing of samples using the gold standard functional virology assay in which evidence of replication-competent, potentially infectious virus particles is obtained by looking for evidence of cytopathic effect (killing) in what are known as permissive cells (i.e, cells that are stripped of their antiviral properties so that viruses can readily infect them).
This was done by Canada’s National Microbiology Laboratory, with the CT cut-off determined to be only 24, meaning that tests showing positive results at CT values >24 failed to demonstrate the presence of potentially infectious viral particles.
Further, the presence of replication-competent viral particles in a sample does not necessarily equate to a case of COVID-19. The latter can only be defined if an active infection is present in conjunction with signs and/or symptoms of illness; the latter would require assessment by a physician.
Remarkably, however, places like Public Health Ontario have been categorizing samples with CT cut-offs of up to 38 cycles and, in some cases, an absence of clinical data, as representing positive cases of COVID-19. This is preposterous, especially in the absence of publicly available data proving that the CT cut-off was established using the gold standard functional virology assay.
Consequently, cases of COVID-19 have likely been dramatically overestimated, but to an unknown degree. Overestimation of the problem of COVID-19 has resulted in unnecessary pressures to force COVID-19 vaccines on individuals.