21 Surveys of Side Effects Following Vaccination Showing Shocking Rates of *SEVERE* Adverse Events
From heart conditions to menstrual irregularities, it's not only the rate of systemic side effects that's stratospheric
I wrote an earlier version of this article, but it did not include text descriptions of the highlighted data. There are also a few other additions or clarifications added to this version.
Although the establishment has been forced to concede that many of the injuries long denied to have any association to the covid vaccines can indeed be caused by the covid vaccines, they still insist that they are exceedingly rare.
The following compilation of studies are all surveys of a defined cohort of vaccine recipients where the rates of severe adverse events - or something that is a good proxy for a severe adverse event - are orders of magnitude greater than the proffered “exceedingly rare” rates in the ballpark of 1/100,000 proffered by the medical establishment.
For each study I am including the title, link, the highlights, and a screenshot/s of the highlighted data in the study (if one is readily available); I also included an excerpt from the study text if it directly described highlighted results.
At the end of the article there is a more detailed explanation of the virtues and limitations of these studies.
One point before we begin, 0.1% = 1/1000. If 1/1000 vaccine recipients suffer a major adverse event, that would mean worldwide approximately 5.5 MILLION people suffered this severe adverse event (5.5 billion vaccinated people / 1000) - and this is only for the ONE adverse event that is showing up as .1%.
1% = 1/100 people, extrapolated worldwide this would equal 55 MILLION people;
2% = 1/50 people, extrapolated worldwide this would equal 110 MILLION people;
5% = 1/20 people, extrapolated worldwide this would equal 275 MILLION people;
suffering just ONE severe adverse event as a consequence of getting vaccinated.


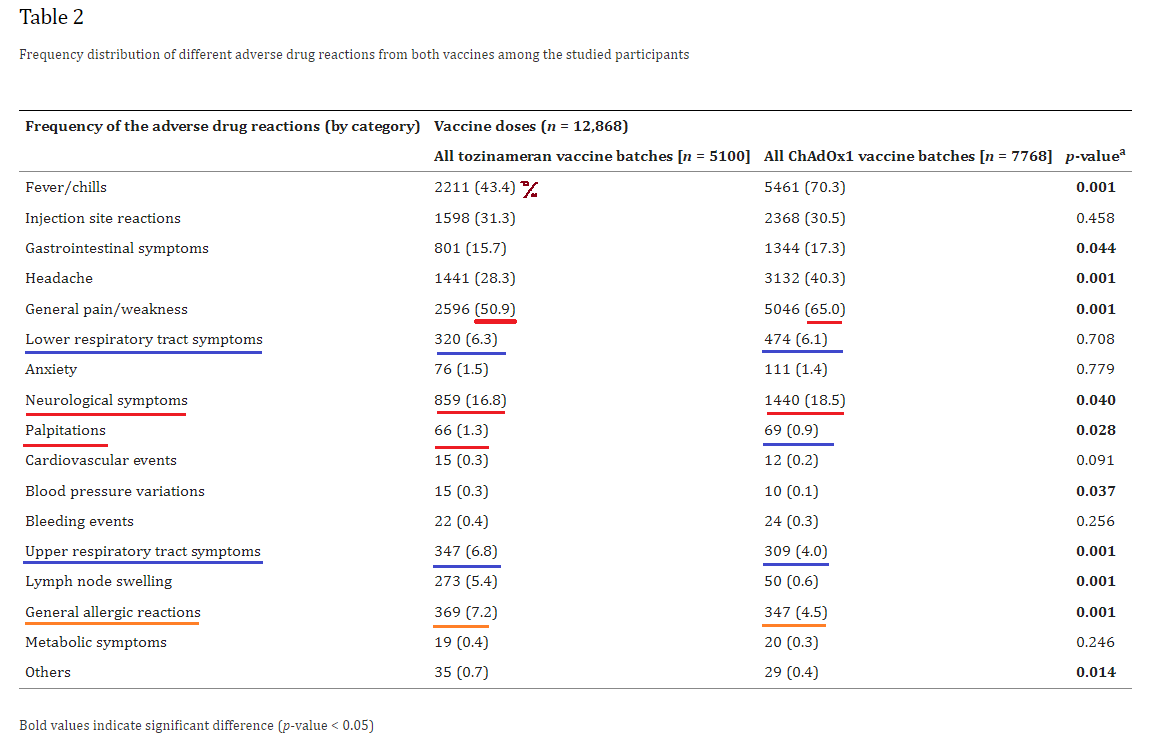
1. Adverse drug reactions from two COVID-19 vaccines reported in Saudi Arabia
https://pubmed.ncbi.nlm.nih.gov/35095267
Highlights:
16.8% of Pfizer recipients, and 18.5% of AstraZeneca recipients, suffered neurological symptoms
1.5% of Pfizer, and 1.4% of AZ, suffered anxiety (the vaccines can cause psychosis and other alterations of mental/emotional state)
1.3% of Pfizer, and 0.9% of AZ, suffered heart palpitations (indicating likely cardiac damage of some sort)
7.2% of Pfizer, and 4,5% of AZ, suffered an allergic reaction
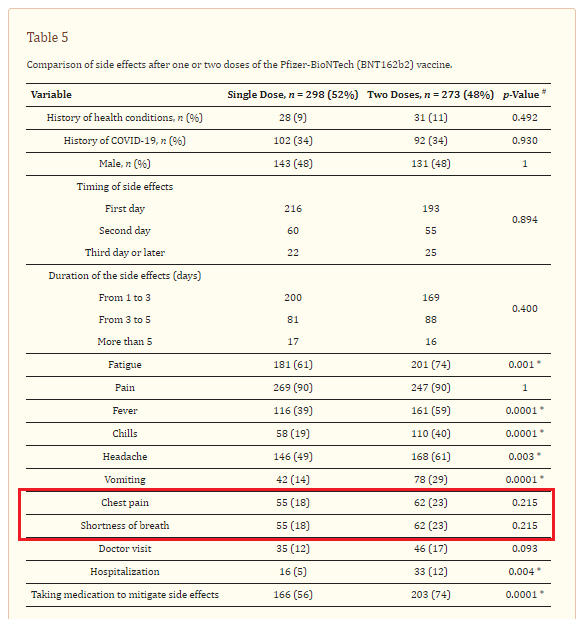
2. Side Effects of COVID-19 Pfizer-BioNTech mRNA Vaccine in Children Aged 12–18 Years in Saudi Arabia
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8621258/
Highlights:
18% of Pfizer recipients experienced shortness of breath and chest pain after dose 1
23% of Pfizer recipients experienced shortness of breath and chest pain after dose 2
5% of Pfizer recipients were hospitalized following dose 1
12% of Pfizer recipients were hospitalized following dose 2
Results: The study recruited a total of 965 eligible participants. Overall, 571 (60%) of the study participants reported at least one side effect following Pfizer-BioNTech (BNT162b2) mRNA vaccination. The most frequently reported side effects were pain or redness at the site of injection (90%), fatigue (67%), fever (59%), headache (55%), nausea or vomiting (21%), and chest pain and shortness of breath (20%). Joint or bone pain were reported less frequently among our participants (2%). Our data showed that more female participants reported side effects compared to male participants, with 52% and 48%, respectively. Side effects were more common after the second dose compared to the first dose in our study cohort.
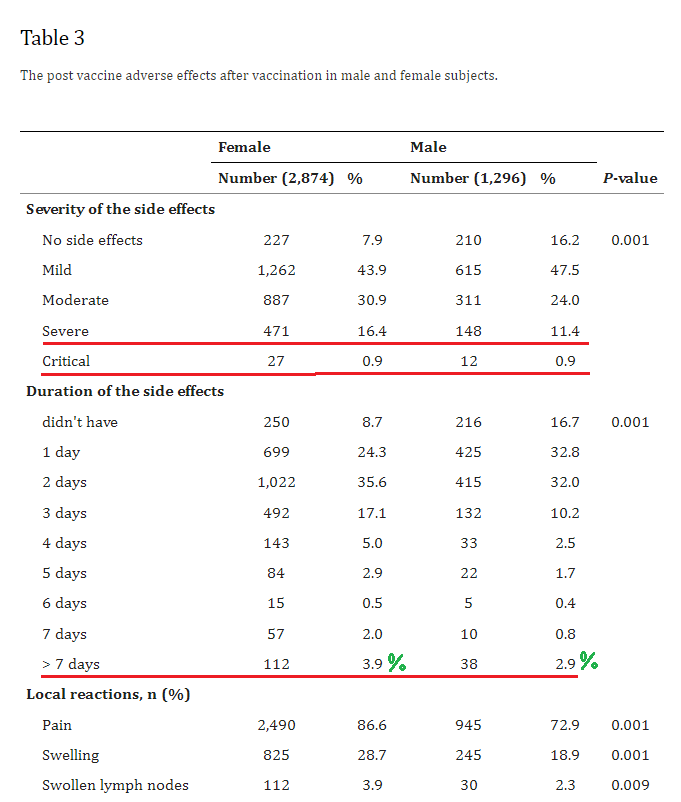
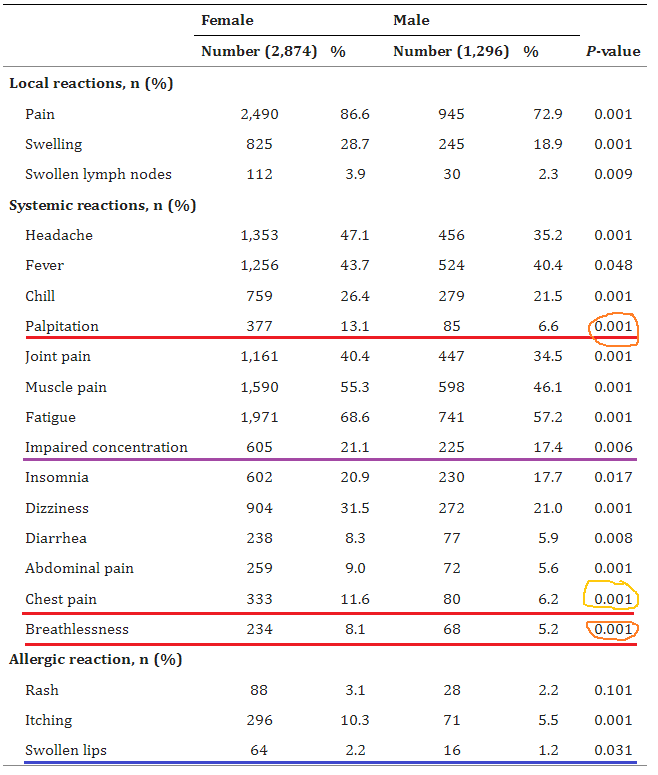
3. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-vaccination Side-Effects Among Saudi Vaccinees
https://pubmed.ncbi.nlm.nih.gov/34692740/
Highlights:
13.1% of females / 6.6% of males, experienced heart palpitations
11.6% females / 6.2% males, experienced chest pain
8.1% females / 5.2% males, experienced shortness of breath
21.1% of females / 17.4% of males, experienced “impaired concentration”
4. Evaluation of Short-Term Symptoms Associated With COVID-19 Vaccines Used Among Adolescents in Saudi Arabia
Highlights:
2.3% of vaccine recipients were hospitalized
Side effects reported after getting the COVID-19 vaccine
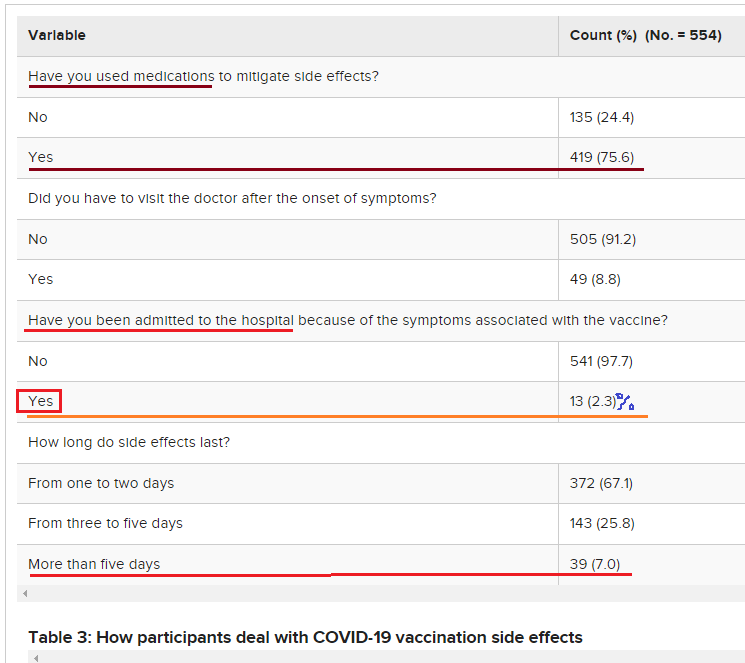
Among participants who experienced COVID-19 vaccination adverse reactions (54.9%, No.= 554), 87.5% had pain at the site of injection, 84.5% reported fatigue, 69% had a headache, 67.5% had a fever, 39.7% had chills, and 19.1% had nausea and vomiting (Figure 1). Other adverse effects had been recorded by the participants such as menstrual disturbance, lymph node enlargement, muscle and bone aches, runny nose, red eye, flu, and drowsiness. Of note, 75.6% of the participants reported using medications to avoid or mitigate vaccination side effects; however, about 9% of those participants had to visit the doctor after the onset of symptoms, and only 2.3% needed admission to the hospital because of the symptoms associated with the received vaccine. The vaccine side effects lasted from one to two days in 67.1%, three to five days in 25.8%, and more than five days in 7.0% of the participants (Table 3).
5. Study of the Side Effects of Pfizer and Oxford COVID-19 Vaccines in the Eastern Province of Saudi Arabia
https://pubmed.ncbi.nlm.nih.gov/36196371/
Highlights:
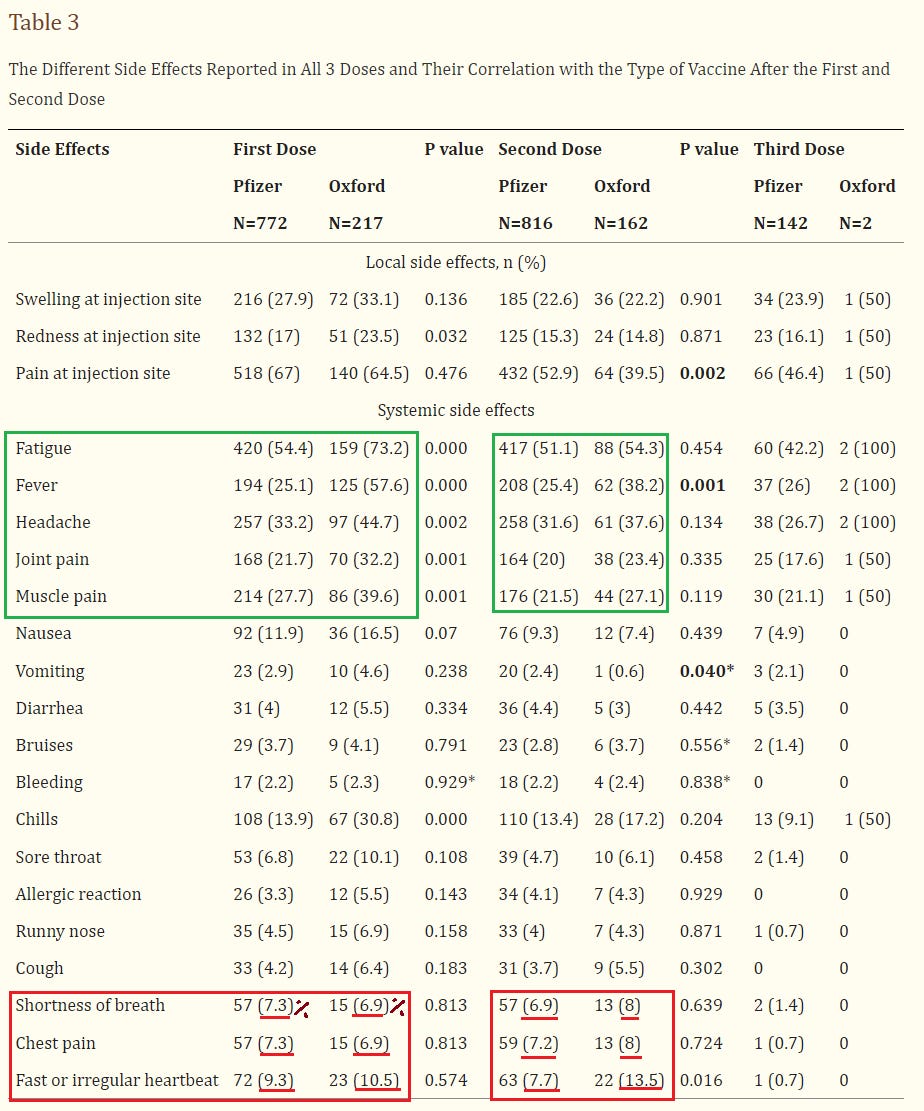
Following Pfizer dose 1, 9.3% experienced irregular heartbeat; 7.3% chest pain; and 7.3% shortness of breath
Following Pfizer Dose 2, 10.5% experienced irregular heartbeat; 6.9% chest pain; & 6.9% shortness of breath
6. Prevalence of side-effects associated with the booster dose of Pfizer-BioNTech (BNT162b2) of COVID-19 Vaccine among vaccinated adults in the Eastern province of Saudi Arabia
https://pubmed.ncbi.nlm.nih.gov/36276167/
Highlights:
Following a Pfizer booster:
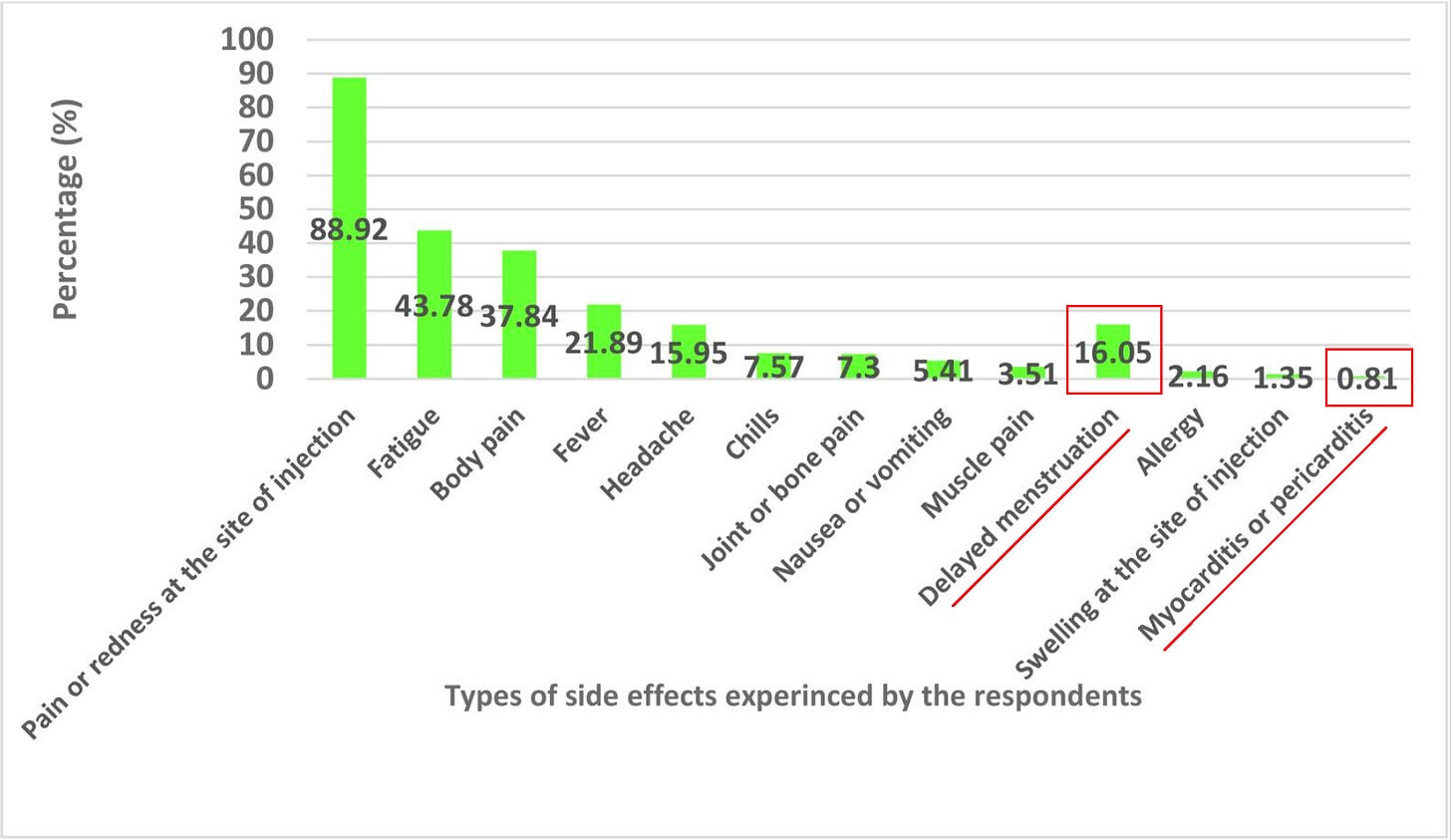
0.81% reported myo/pericarditis (!!!) (which would have to be clinically diagnosed)
16% reported delayed menstruation
1.1% were hospitalized
Conclusions
Our study showed that participants in the Eastern province of Saudi Arabia receiving a booster dose of the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine commonly experienced short-lived side effects, especially pain or redness at the infection site, but also fatigue, body pain, fever and headache. 2.4% of recipients had sought medical attention for their side effects, although only four participants (1.1%) were admitted to the hospital after experiencing side effects. Findings from this study support the vaccine's safety as well as provide important baseline data for healthcare workers and the general public to be aware of possible side effects following the COVID-19 vaccine. In light of the current study, its results support the vaccine's safety and provide important baseline data that will raise healthcare workers' and the general public's awareness of COVID-19 vaccine expected side-effects. In this way, vaccine-hesitant individuals and pessimists might be convinced to accept the COVID-19 booster dose.
The authors’ contentions of vaccine safety despite the alarming rate of SAE’s they themselves documented here is worth repeating:
“Findings from this study support the vaccine's safety”
“In this way, vaccine-hesitant individuals and pessimists might be convinced to accept the COVID-19 booster dose”
7. Cognizance, adverse effects and motivation regarding COVID-19 vaccination amongst health care professionals: A cross-sectional study
https://dmp.umw.edu.pl/pdf/2022/59/1/13.pdf
Highlights:
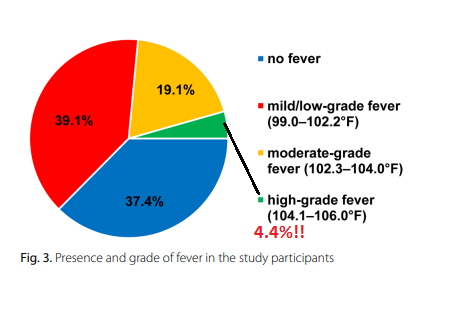
4.4% experienced a fever that was ABOVE 104
8. Investigating trends in those who experience menstrual bleeding changes after SARS-CoV-2 vaccination
https://www.medrxiv.org/content/10.1101/2021.10.11.21264863v2
Highlights:
42% who had regular menstrual cycles experienced heavier bleeding
66% of post-menopausal people reported breakthrough bleeding (!!!!!)
71% of people on long-acting reversible contraceptives reported breakthrough bleeding
Abstract
Early in 2021, many people began sharing that they experienced unexpected menstrual bleeding after SARS-CoV-2 inoculation. We investigated this emerging phenomenon of changed menstrual bleeding patterns among a convenience sample of currently and formerly menstruating people using a web-based survey. In this sample, 42% of people with regular menstrual cycles bled more heavily than usual while 44% reported no change after being vaccinated. Among respondents who typically do not menstruate, 71% of people on long-acting reversible contraceptives, 39% of people on gender-affirming hormones, and 66% of post-menopausal people reported breakthrough bleeding. We found increased/breakthrough bleeding was significantly associated with age, systemic vaccine side effects (fever, fatigue), history of pregnancy or birth, and ethnicity. Generally, changes to menstrual bleeding are not uncommon nor dangerous, yet attention to these experiences is necessary to build trust in medicine.
9. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8206586/
Highlights:
Following Dose #1, 6.19% experienced tachycardia; 5.3% experienced a rise in blood pressure
Following Dose #2, 2.65% experienced tachycardia; 1.8% experienced a rise in blood pressure
Adverse reactions occurred in 87% of subjects after the first dose and 83% after the second dose of vaccine. Most reactions were mild and none of the subjects discontinued normal daily activities. There was transitory loss of taste and smell in 1 subject. Body temperature >38.0 °C, tachycardia, or a rise in BP occurred in 13% and 27% of subjects after the first and second dose, respectively.
The subjects with documented infection by SARS-COV-2 over the previous year showed a higher frequency of systemic reactions to vaccine when compared with those without history of documented infection (38% vs 10%, p = 0.004).
Overall, 6 subjects (5.3%) showed an average rise in systolic or diastolic BP at home by ≥ 10 mmHg during the first 5 days after the first dose of vaccine when compared with the five days before the vaccine. Table 1 shows the main characteristics of these subjects. The BP rise required an intensification of BP-lowering treatment in 4 subjects. Two of the subjects with a BP rise after the first dose experienced a BP rise also after the second dose. Of note, history of COVID-19 was associated with a higher incidence of rise in BP when compared with subjects without previous exposure to SARS-CoV-2 (23% vs 3%, p = 0.002).
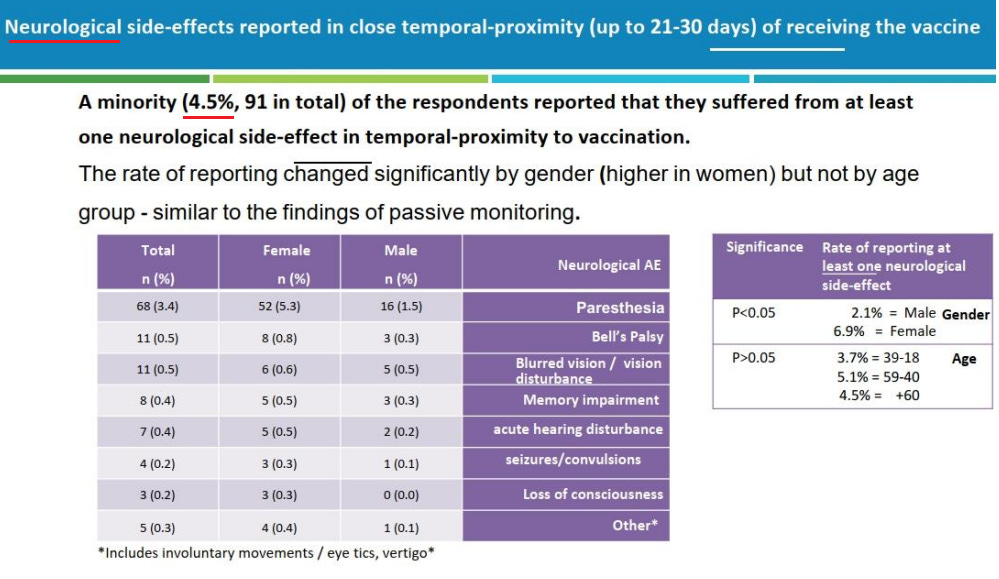
10. Israel Ministry of Health Pfizer Booster Survey
Hebrew: https://drive.google.com/file/d/1NyMrHRTO-SLvygWtPmA39QlgqCE_GbsP/view
English: https://t.me/MOHreport/9197
Highlights:
4.5% experienced a neurological side effect following a booster
3.4% experienced paresthesia
17.1% experienced shaking
5.5% experienced chest pain
11. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan
https://pubmed.ncbi.nlm.nih.gov/35058126/
Highlights:
1% following Pfizer dose 1, and 1.6% following Pfizer dose 2, reported suffering from an adverse event not included in the survey questionnaire
3.6% following Moderna dose 1, and 4.2% following Moderna dose 2, reported suffering from an adverse event not included in the survey questionnaire
Following Pfizer Dose 1, 2.4% suffered a skin rash; 39.9% suffered muscle or joint pain
Following Pfizer Dose 2, 3.4% suffered a skin rash; 49.8% suffered muscle or joint pain
Following Moderna Dose 1, 5.3% suffered a skin rash; 52.3% suffered muscle or joint pain
Following Moderna Dose 1, 6.5% suffered a skin rash; 59.7% suffered muscle or joint pain
12. Adverse Events Following COVID-19 Vaccination in Rivers State, Nigeria: A Cross-Sectional Study
https://pubmed.ncbi.nlm.nih.gov/35488575/
Highlights:
3% were hospitalized following vaccination
1.5% suffered a life-threatening condition following vaccination
Results: In this study, 50.5% of respondents reported post-vaccination adverse events out of which 10 (4.6%) were severe (30% of the severe cases were life-threatening, 60% were hospitalised and 10% were placed on bed rest). The most common side effects were fever (73.0%), pain at the injection site (41.2%), fatigue (33.3%), body ache (17.5%) and headache (13.8%).
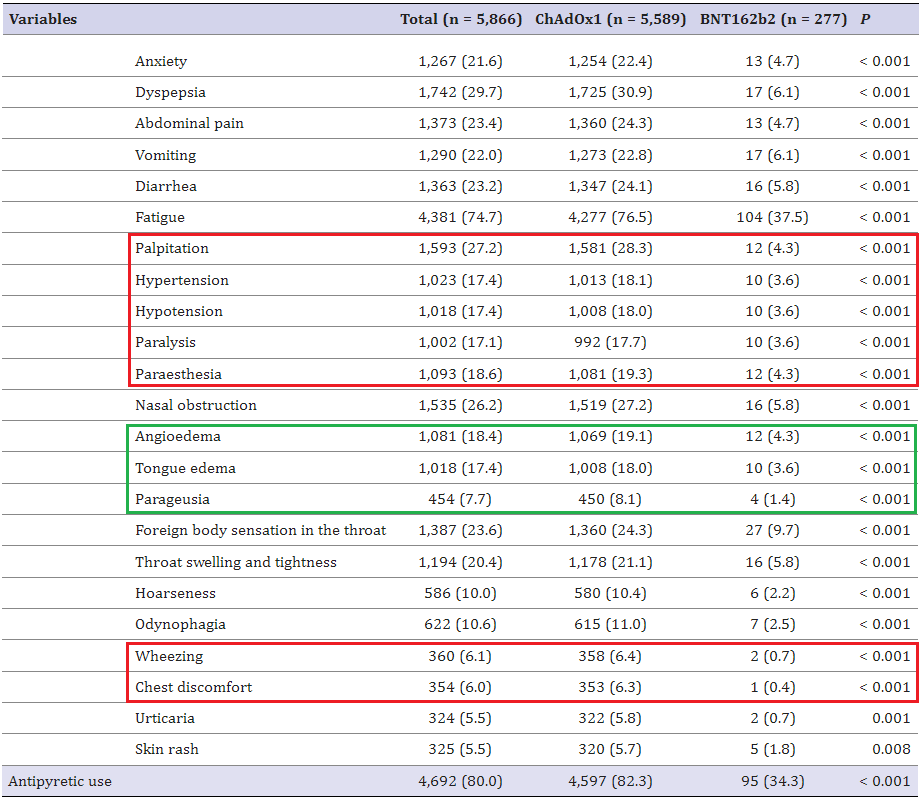
13. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea
https://pubmed.ncbi.nlm.nih.gov/33942579/
Highlights:
Following vaccination with AstraZeneca, 28.3% experienced heart palpitations; 18.1% hypertension; 18% hypotension; 17.1% paralysis; 18.6% paresthesia; 6.4% wheezing (shortness of breath); 6.3% chest pain
Following Pfizer vaccination, 4.3% experienced heart palpitations; 3.6% hypertension; 3.6% hypotension; 36% paralysis; 4.3% paresthesia; 0.7% wheezing; 0.4% chest pain
14. Side effects of the Pfizer BioNTech vaccine in health workers of a hospital in the southeast of Mexico
https://pubmed.ncbi.nlm.nih.gov/36223615/
Highlights:
6.14% experienced cheat pain following vaccination
8.9% experienced hypertension following vaccination
15. Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021
https://pubmed.ncbi.nlm.nih.gov/34819146/
Highlights:
13% of vaccine recipients were unable to go to work the next day following vaccination
7.52% went to the doctor
0.72% were hospitalized
Results
A total of 1,639 health professionals (70.2% male) with a median age of 32 (interquartile range: 27-40) were enrolled. At least one adverse event was reported among 71.6% of participants (95% CI = [69.3-73.8]). The most commonly reported adverse events were injection site pain (91.0%), asthenia (74.3%), headache (68.7%), soreness (55.0%), and fever (47.5%). An increased libido was also reported in 3.0% of participants. Of the participants who experienced adverse events, 18.2% were unable to go to work the day after vaccination, 10.5% consulted a medical doctor, and 1.0% were hospitalized. The SAEs’ prevalence was 23.8% (95% CI = [21.8-25.9]). Being <30 years (AOR = 5.54; p<0.001), or 30-49 years (AOR = 3.62; p<0.001) and being female (AOR = 1.97; p<0.001) were associated with SAEs.
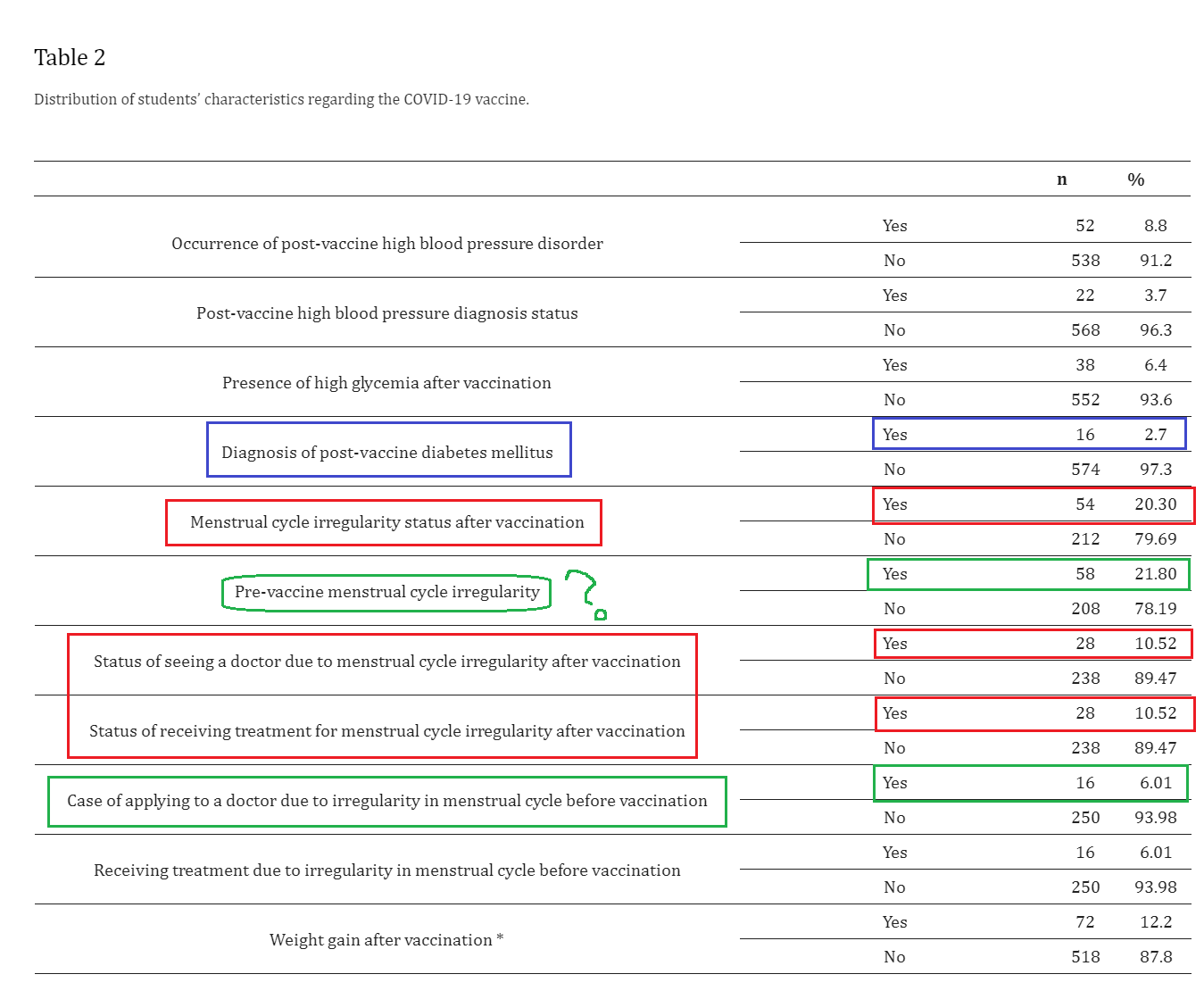
16. Determining the Health Problems Experienced by Young Adults in Turkey, Who Received the COVID-19 Vaccine
https://pubmed.ncbi.nlm.nih.gov/36146604/
I wrote up a partial explanation of this study’s findings here.
Highlights:
Of 590 undergraduate university students surveyed:
2.7% of vaccine recipients were newly diagnosed with diabetes
10.52% of females suffered a menstrual issue severe enough to require medical intervention shortly after vaccination
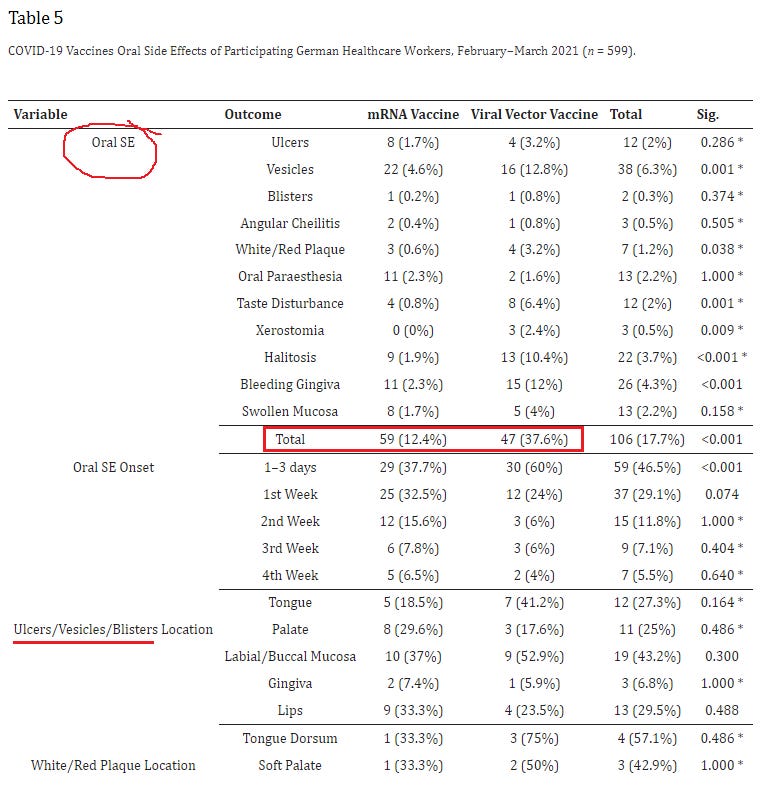
17. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers
https://pubmed.ncbi.nlm.nih.gov/34439984/
Highlights:
12.4% suffered an oral injury following Pfizer vaccination; 37.6% following AZ
2.3% suffered paresthesia following Pfizer vaccination; 1.6% following AZ
3.7% of females suffered a Skin condition following Pfizer (compared to 0 males)
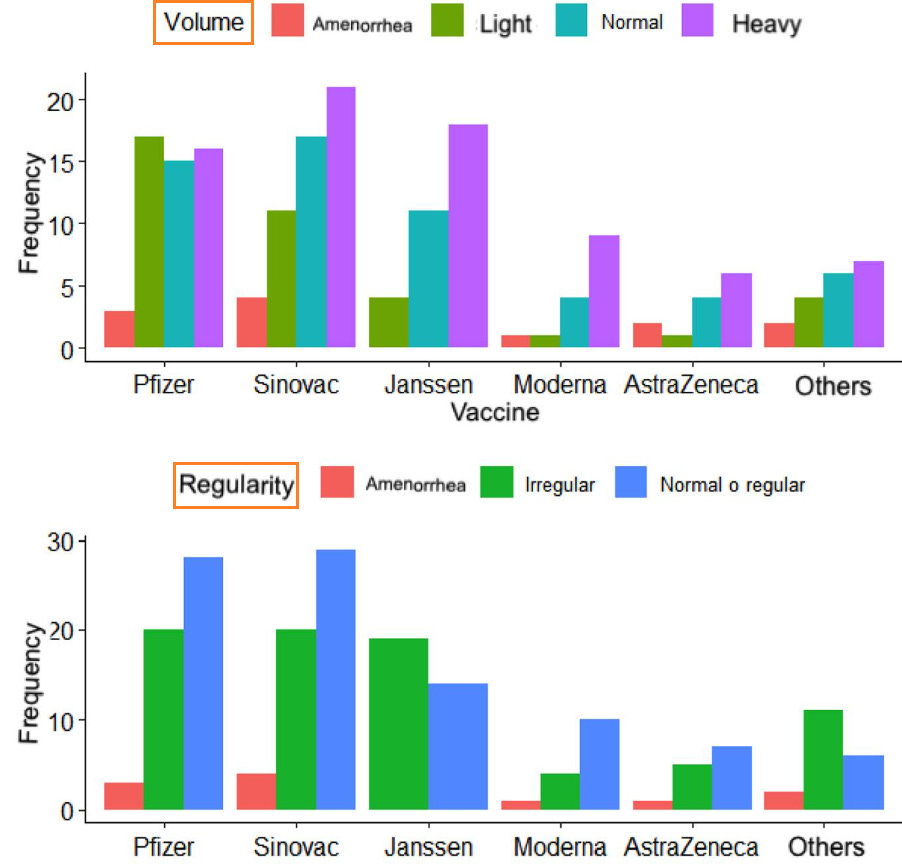
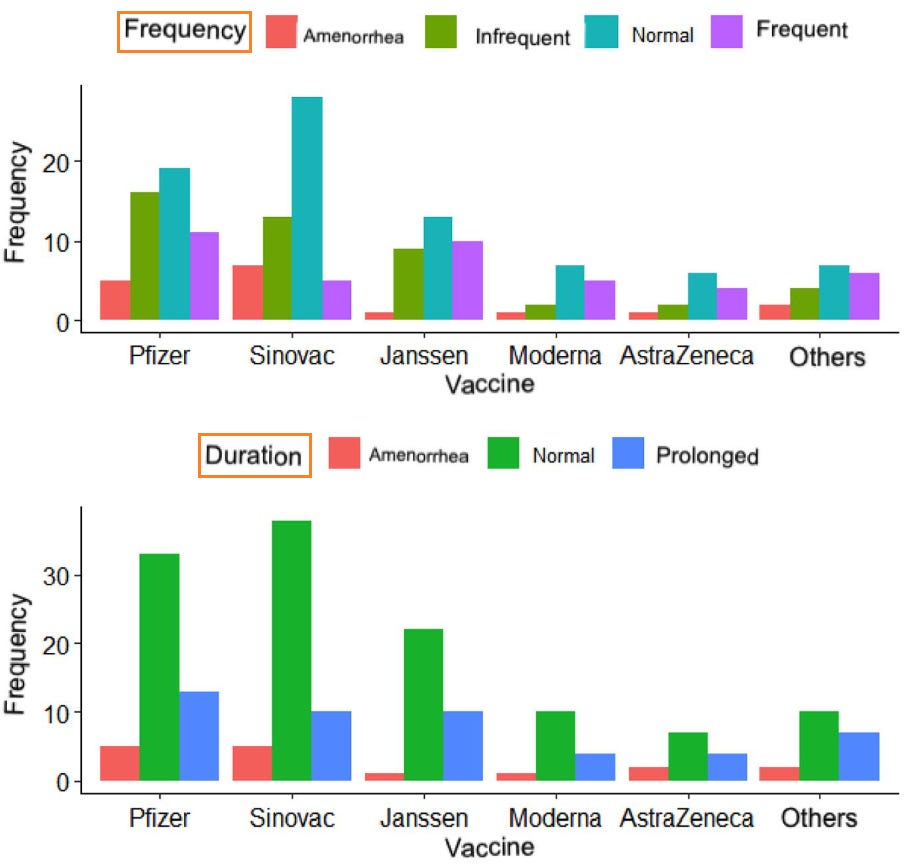
18. Menstrual cycle disturbances after COVID-19 vaccination
https://pubmed.ncbi.nlm.nih.gov/35796571/
Highlights:
42.93% of women reported irregular menstrual cycles post-vaccination
5.97% reported amenorrhea (missing their periods altogether)
26.08% reported longer duration of bleeding
31.36% reported decreased quality of life after vaccination
Post-vaccination changes: regularity
Overall, 94 women (51.08%) reported regular cycles after vaccination, 79 (42.93%) noted irregular cycles after vaccination (variation > 9 days), and 11 (5.97%) of them reported having amenorrhea after vaccination. The vaccine that most frequently affected cycle regularity was Pfizer and Sinovac; however, for the J&J/Janssen vaccine more women reported irregular cycles (n = 19) than normal (58% versus 42%).
Post-vaccination changes: duration
In relation to the duration of the menstrual cycle, 120 women (65.21%) mentioned a duration in normal ranges (<8 days), 26.08% (48) commented a prolongation of the cycle (>9 days), and only 16 women (8.69%) reported amenorrhea/absent.
Post-vaccination changes: volume
The volume variable was reported as abnormal by 127 women (69.02%), being heavy in 41.84% (n = 77), light in 20.65% (n = 38), and absent in 6.52% (n = 12). Overall, 30.97% (n = 57) of the women described the volume as normal. When discriminating by biologics, 17 women (9.23%) vaccinated with Pfizer reported light volume, while the others reported predominantly heavy cycles (J&J/Janssen n = 18, Sinovac n = 21, Moderna n = 9, others n = 7, AstraZeneca n = 6).
Affectation in the quality of life
Of the 950 women who answered the survey, 31.36% (n = 298) commented that their QoL was affected after vaccination. Likewise, of the 184 who reported alterations in their menstrual cycle, 55.97% (103) stated a deterioration in their QoL after having been vaccinated in all the standard indicators evaluated perception of wealthiness, physical and mental health, pain.
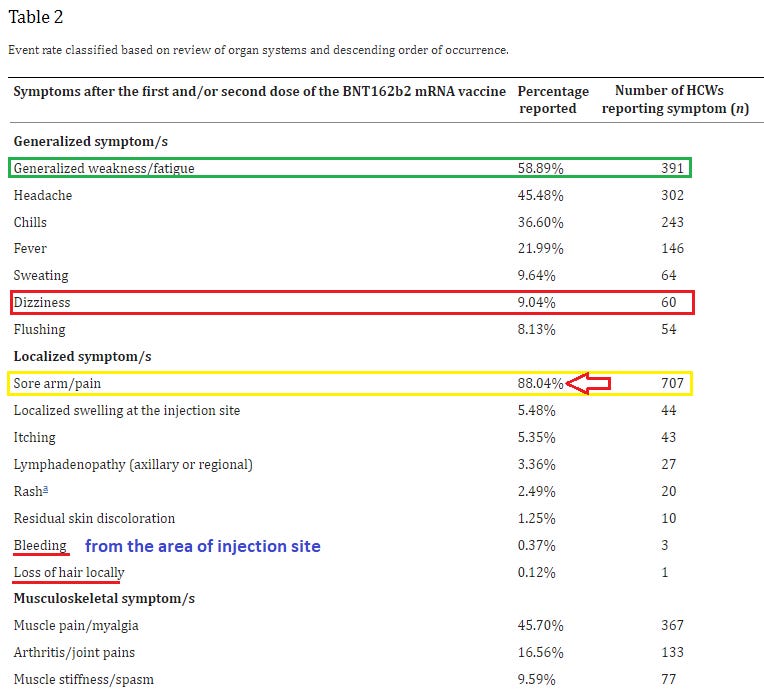
19. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers
https://pubmed.ncbi.nlm.nih.gov/33866000/
This study in particular shows it’s amazing what you can find out when you just ask - this is an impressive amount of individual side effects that you won’t find in almost any other survey or study of vaccine ‘safety’.
Furthermore, this is a survey of healthcare workers, who are better trained to be observant of, & be able to describe with technical precision, clinical changes or side effects that occur, lending more credence than merely surveying a bunch of random laypeople who are more easily confused, susceptible to suggestion, and less precise in their observations and recall of clinical symptoms.
Highlights:
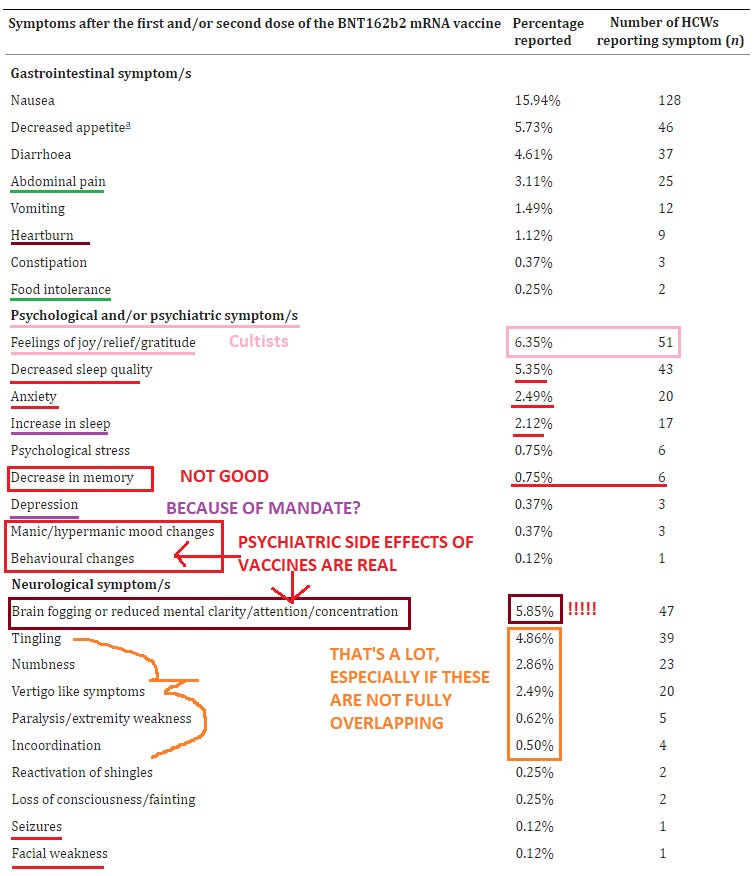
Psychological: 9.04% suffered dizziness after vaccination; 5.85% reported brain fog (!!!); 6.35% reported some form a euphoria following vaccination; 5.35% reported decreased sleep quality; 2.49% reported anxiety; 0.75% reported memory impairment
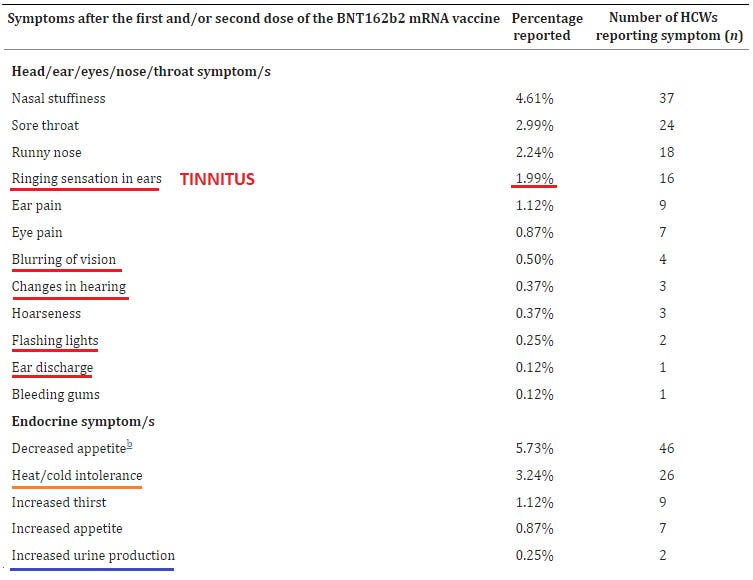
Neurological: 4.86% reported tingling; 2.86% numbness; 2.49% vertigo; 0.62% paralysis / extreme weakness; 0.5% lack of physical coordination; 0.12% seizures; 1.99% tinnitus (!!); 0.5% visual blurring
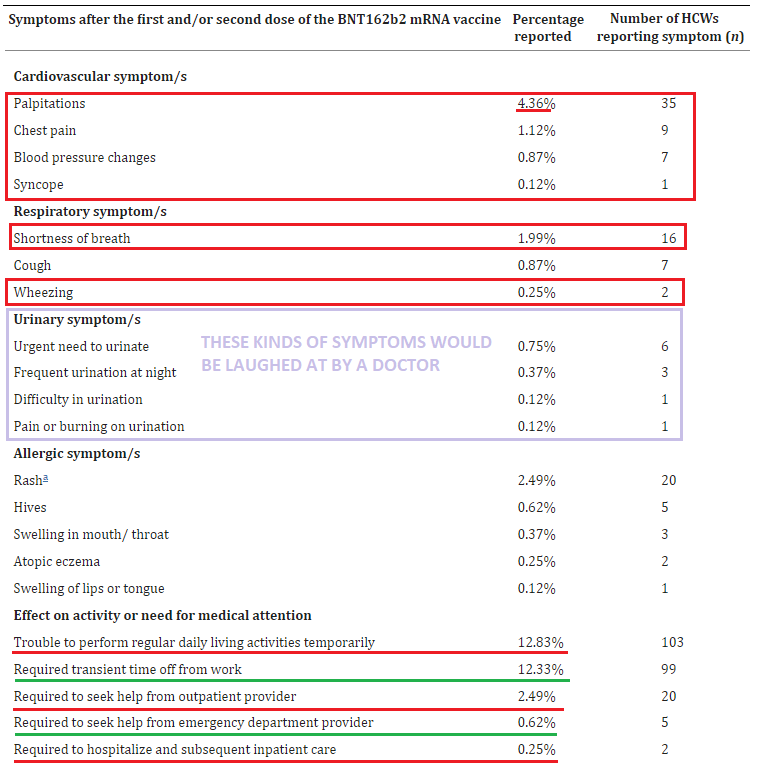
Cardiovascular: 4.36% reported heart palpitations; 1.12% chest pain; 0.87% changes in blood pressure; 1.99% shortness of breath; 0.25% wheezing
0.62% had to go the ER & 0.25% were hospitalized
20. Safety and side effect profile of Pfizer-BioNTech COVID-19 vaccination among healthcare workers: A tertiary hospital experience in Singapore
https://pubmed.ncbi.nlm.nih.gov/34625758/
Highlights:
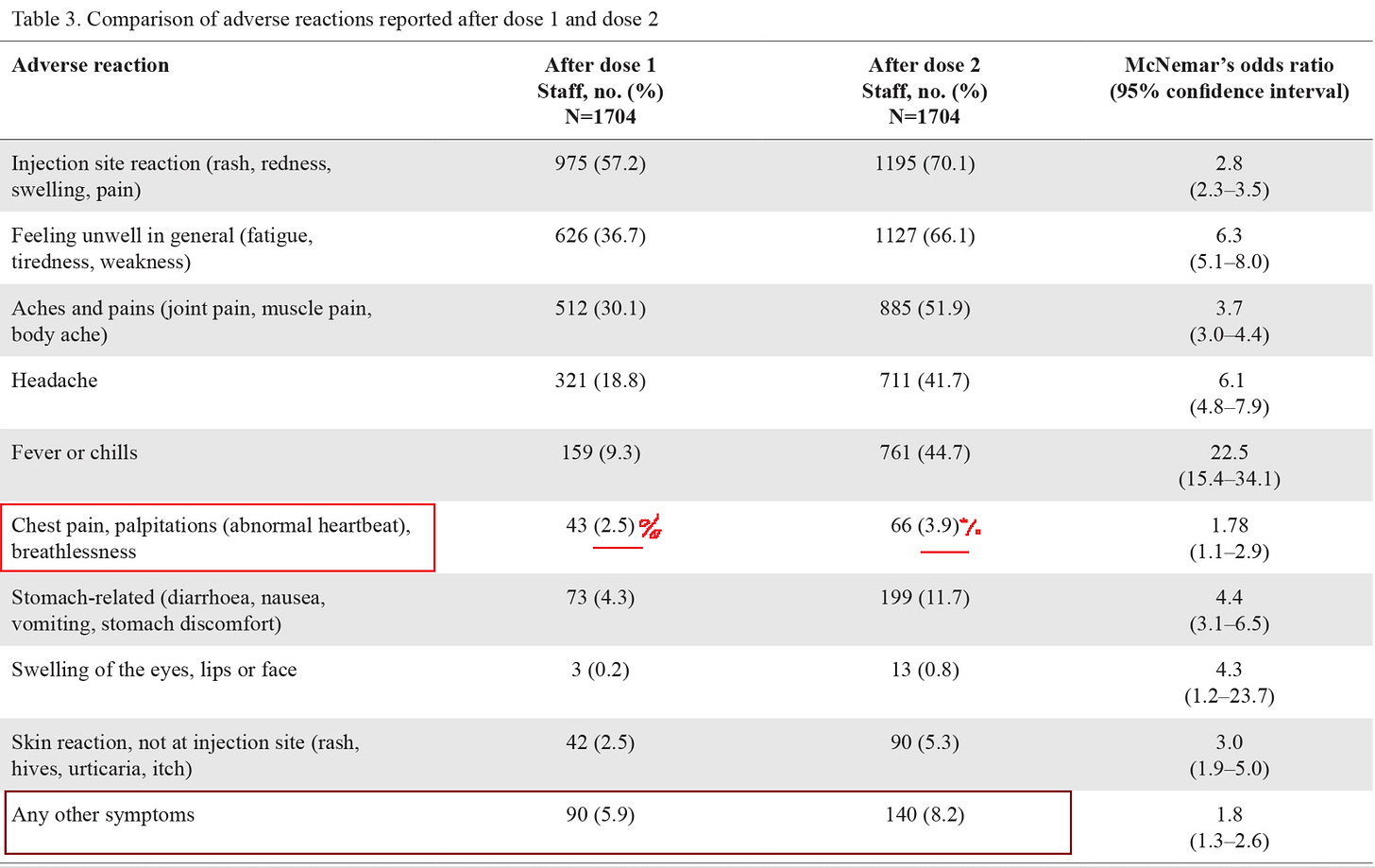
2.5% suffered chest pain, palpitations or shortness of breath following dose 1
3.9% suffered chest pain, palpitations or shortness of breath following dose 2
2.5% suffered a skin condition following dose 1
5.3% suffered a skin condition following dose 2
5.9% suffered an injury not specified in the survey following dose 1
8.2% suffered an injury not specified in the survey following dose 2
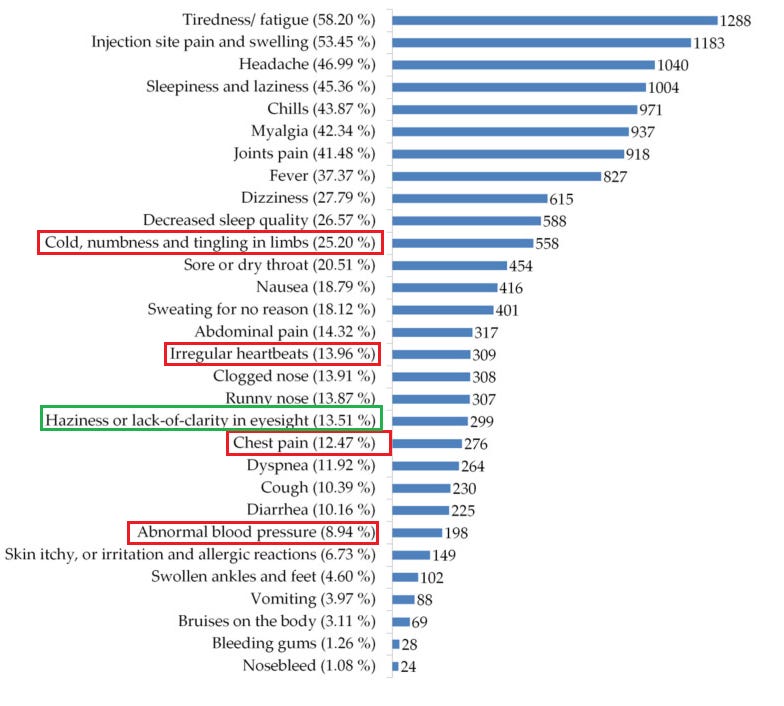
21. Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects
https://pubmed.ncbi.nlm.nih.gov/34073382/
Highlights:
25.2% suffered cold, numbness or tingling
13.96% suffered irregular heartbeat
13.51% suffered a vision impairment
12.47% suffered chest pain
8.94% suffered abnormal blood pressure
Background
This list is partial, I stopped at 21 because it was simply too long to keep adding more.
This is only including formal academic literature.
Obviously, there are a number of technical qualifiers that are relevant to properly parse any study individually. They are not of uniform quality and have widely ranging characteristics. Therefore, one cannot simply run with the topline numbers presented for any individual study and assume that they are definitive proof or statistically accurate by themselves.
However, they are reporting much higher rates of SAE’s then is claimed by the mainstream narrative. (Most severe adverse events will not show up for a few reasons, such as that they are not solicited; people with severe injuries are usually incapacitated and unable to participate in surveys; and the failure or refusal to diagnose severe injuries, among others.)
I am hoping to write future posts to unpack some of these surveys individually, because there is a lot to say about many of them that cannot be included in a massive compilation for brevity’s sake. I did try and highlight the most relevant bits of information from each.
The rate of so-called ‘routine’ side effects (systemic and local) are themselves off the charts - regular medicines and vaccines do not cause anywhere near this degree of unpleasant reactions.








Since we have no clue as to the possibility of long-term effects caused by these horrendous substances, how will this info be accumulated in the years ahead? I suspect there will be many severe reactions that will never be connected to these injections...just as the medical terrorists desire.
Thanks great compilation. I’ve Been doing battle with some Covidians.