21 Surveys of Side Effects Following Vaccination Showing Shocking Rates of *SEVERE* Adverse Events
From heart problems to menstrual irregularities, it's not only the rate of systemic side effects that's stratospheric
“Show me the evidence.”
So demand most people when someone tries to convince that the covid vaccines might actually be responsible for a tremendous amount of medical carnage.
What follows is a compilation of surveys published as formal studies gauging the rates of various side effects or adverse events following vaccination with the 4 vaccines used in Western countries - Pfizer, Moderna, J&J, & AstraZeneca.
In other words, evidence.
A few caveats/notes:
This list is partial, I stopped at 21 because it was simply too long to keep adding more.
This is only including formal academic literature.
Obviously, there are a number of technical qualifiers that are relevant to properly parse any study individually. They are not of uniform quality and have widely ranging characteristics. Therefore, one cannot simply run with the topline numbers presented for any individual study and assume that they are definitive proof or statistically accurate by themselves.
However, they seem to all be in the same ballpark as far as incidence rates for specific types of side effects, which is evidence that they are likely to be generally representative for the portion of vaccine injuries that can be captured with these types of surveys. (Most severe adverse events will not show up for a few reasons, most importantly that they are simply not solicited.)
I am hoping to write future posts to unpack some of these surveys individually, because there is a lot to say about many of them that cannot be included in a massive compilation for brevity’s sake. I did try and highlight the most relevant bits of information from each.
The rate of so-called ‘routine’ side effects (systemic and local) are themselves off the charts - regular medicines and vaccines do not cause anywhere near this degree of unpleasant reactions.
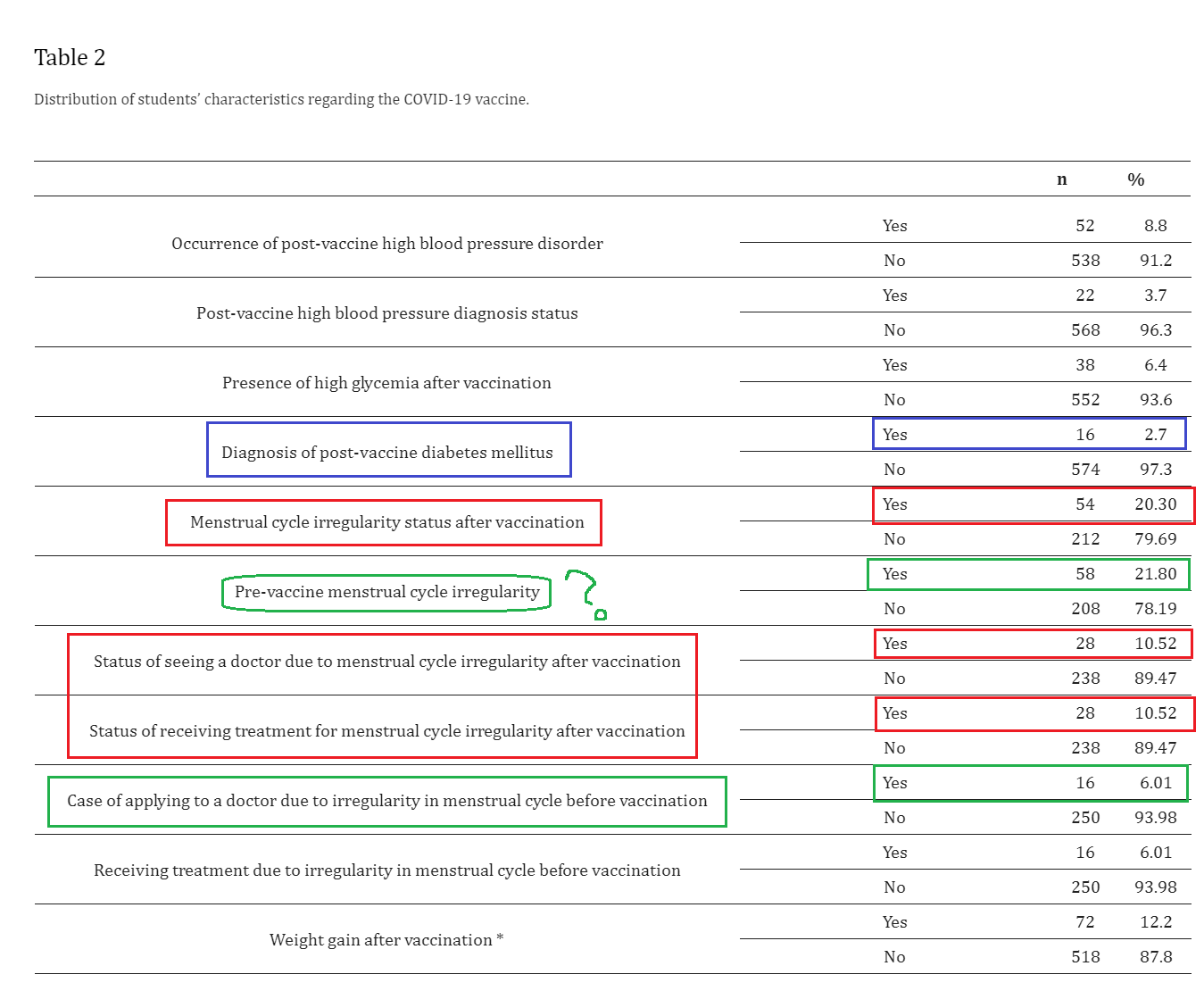
1. Adverse drug reactions from two COVID-19 vaccines reported in Saudi Arabia
https://pubmed.ncbi.nlm.nih.gov/35095267
2. Side Effects of COVID-19 Pfizer-BioNTech mRNA Vaccine in Children Aged 12–18 Years in Saudi Arabia
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8621258/
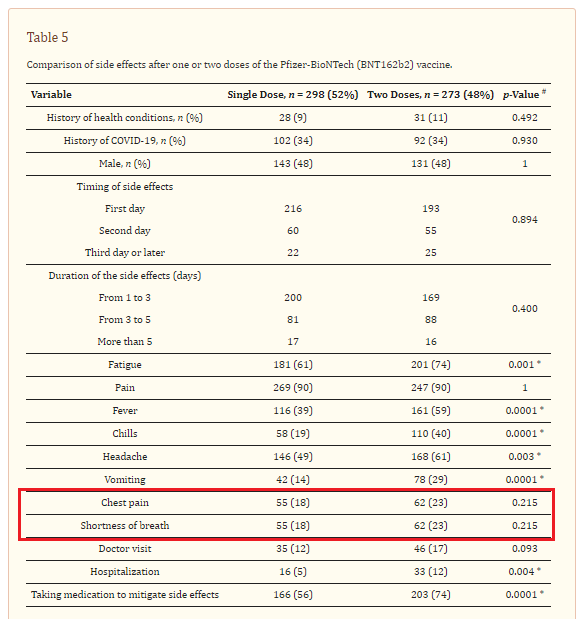
Results: The study recruited a total of 965 eligible participants. Overall, 571 (60%) of the study participants reported at least one side effect following Pfizer-BioNTech (BNT162b2) mRNA vaccination. The most frequently reported side effects were pain or redness at the site of injection (90%), fatigue (67%), fever (59%), headache (55%), nausea or vomiting (21%), and chest pain and shortness of breath (20%). Joint or bone pain were reported less frequently among our participants (2%). Our data showed that more female participants reported side effects compared to male participants, with 52% and 48%, respectively. Side effects were more common after the second dose compared to the first dose in our study cohort.
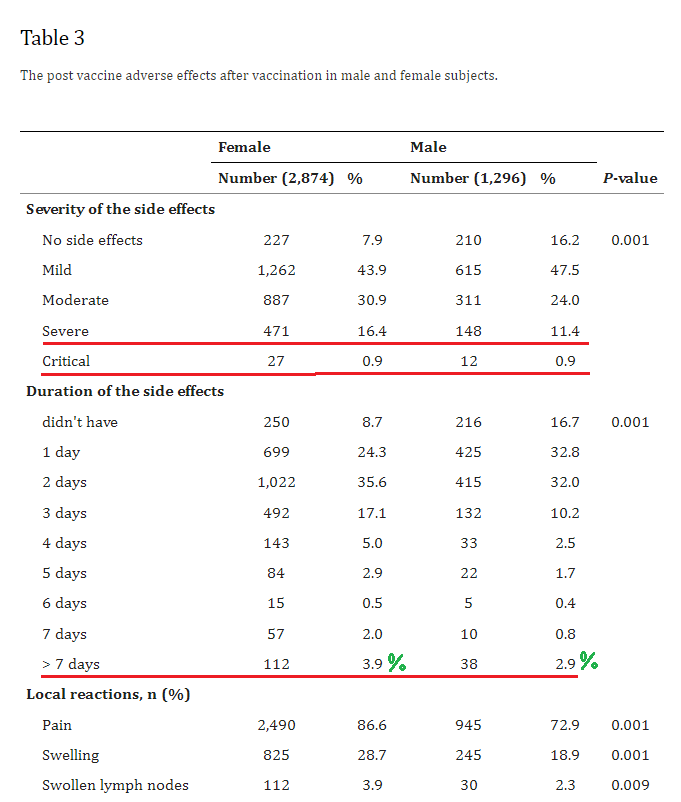
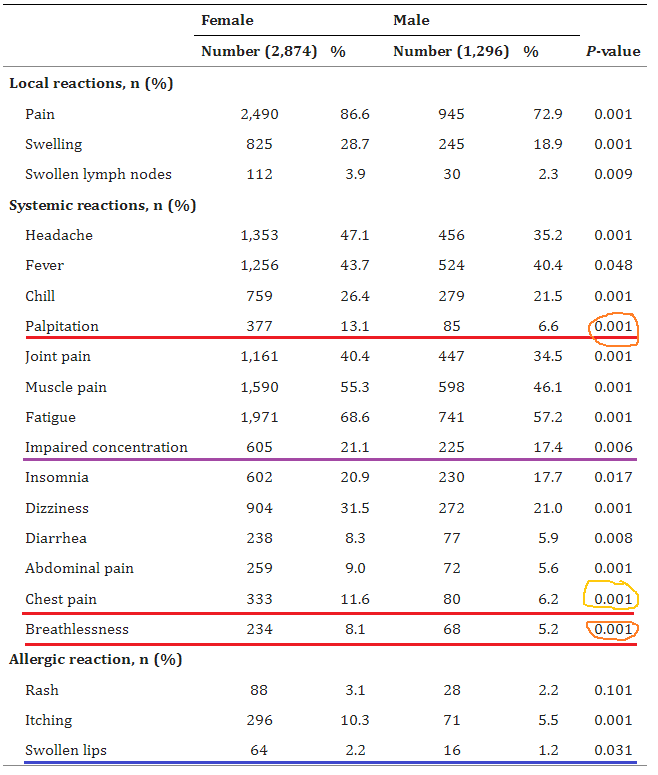
3. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-vaccination Side-Effects Among Saudi Vaccinees
https://pubmed.ncbi.nlm.nih.gov/34692740/
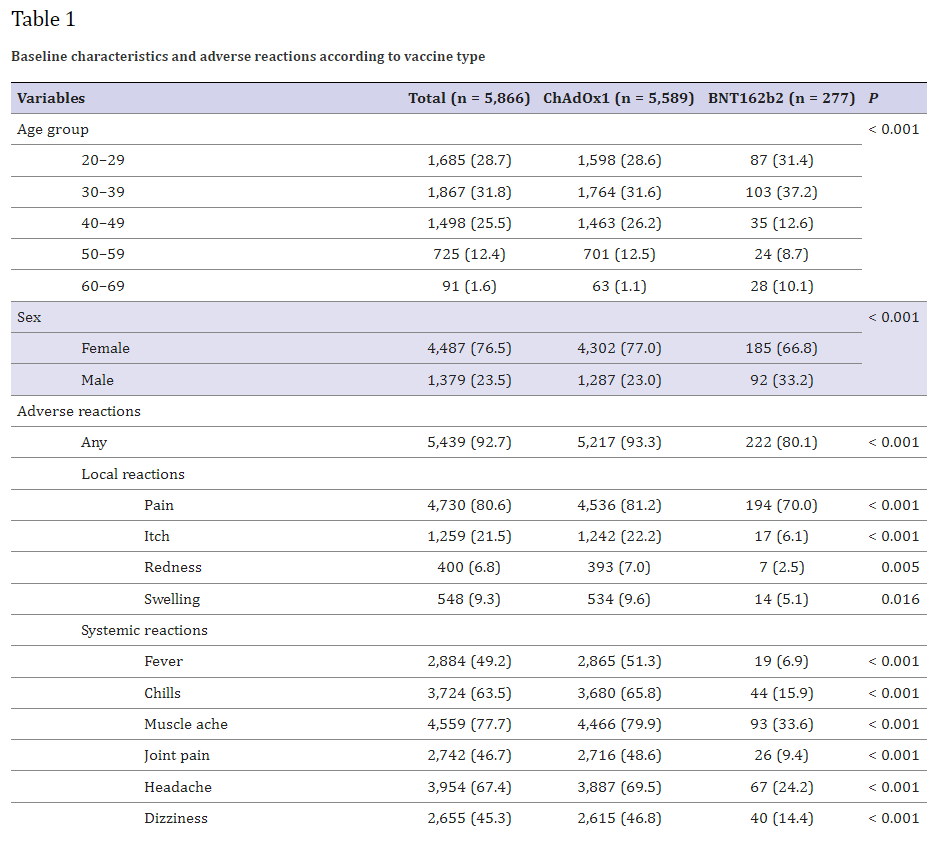
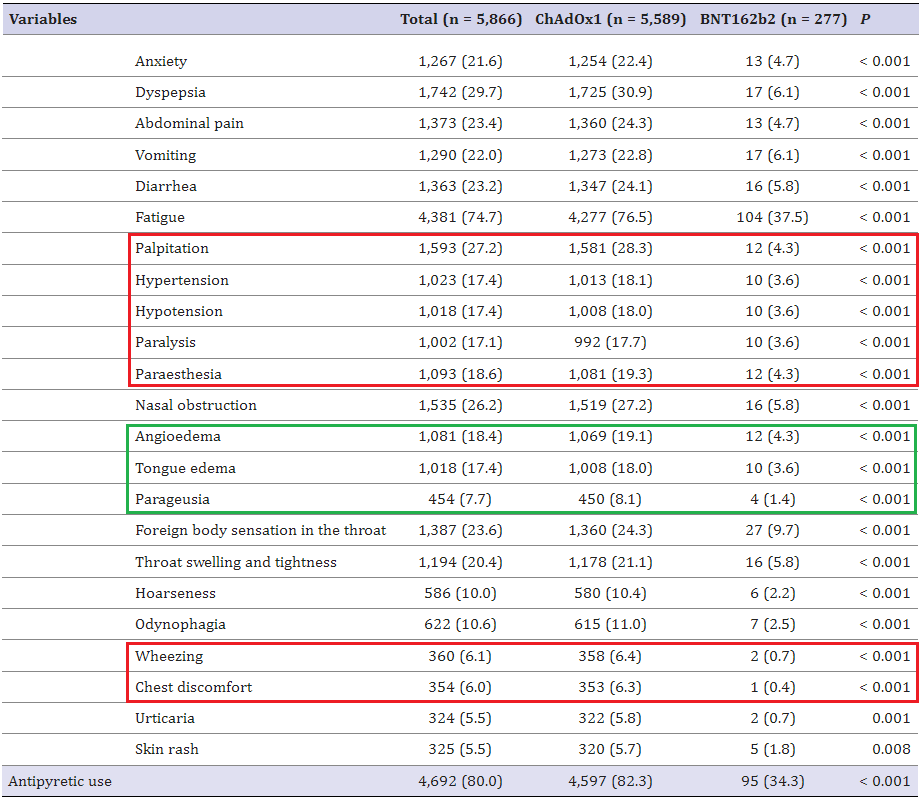
The rates of these severe adverse events are off the charts -
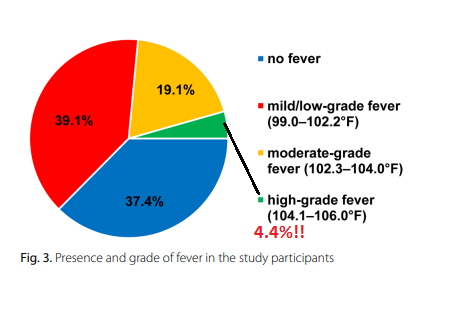
4. Evaluation of Short-Term Symptoms Associated With COVID-19 Vaccines Used Among Adolescents in Saudi Arabia
Side effects reported after getting the COVID-19 vaccine
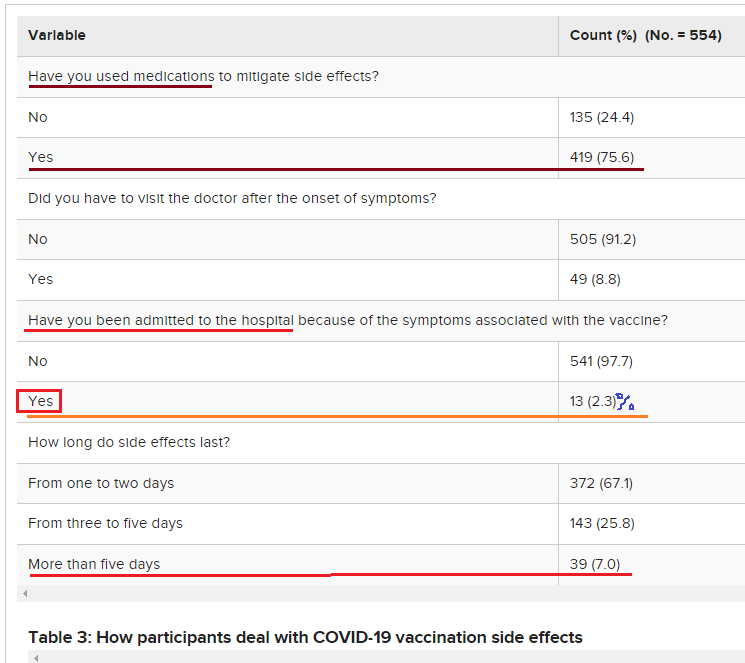
Among participants who experienced COVID-19 vaccination adverse reactions (54.9%, No.= 554), 87.5% had pain at the site of injection, 84.5% reported fatigue, 69% had a headache, 67.5% had a fever, 39.7% had chills, and 19.1% had nausea and vomiting (Figure 1). Other adverse effects had been recorded by the participants such as menstrual disturbance, lymph node enlargement, muscle and bone aches, runny nose, red eye, flu, and drowsiness. Of note, 75.6% of the participants reported using medications to avoid or mitigate vaccination side effects; however, about 9% of those participants had to visit the doctor after the onset of symptoms, and only 2.3% needed admission to the hospital because of the symptoms associated with the received vaccine. The vaccine side effects lasted from one to two days in 67.1%, three to five days in 25.8%, and more than five days in 7.0% of the participants (Table 3).
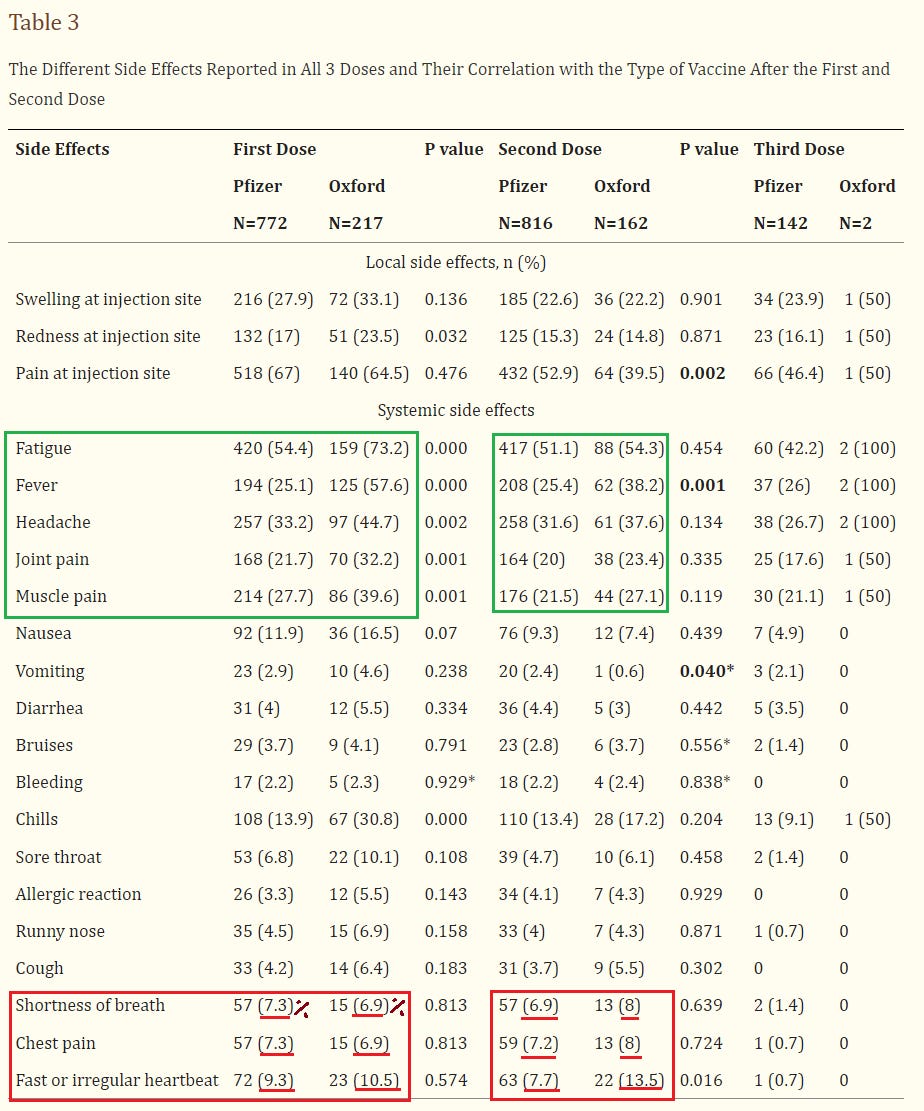
5. Study of the Side Effects of Pfizer and Oxford COVID-19 Vaccines in the Eastern Province of Saudi Arabia
https://pubmed.ncbi.nlm.nih.gov/36196371/
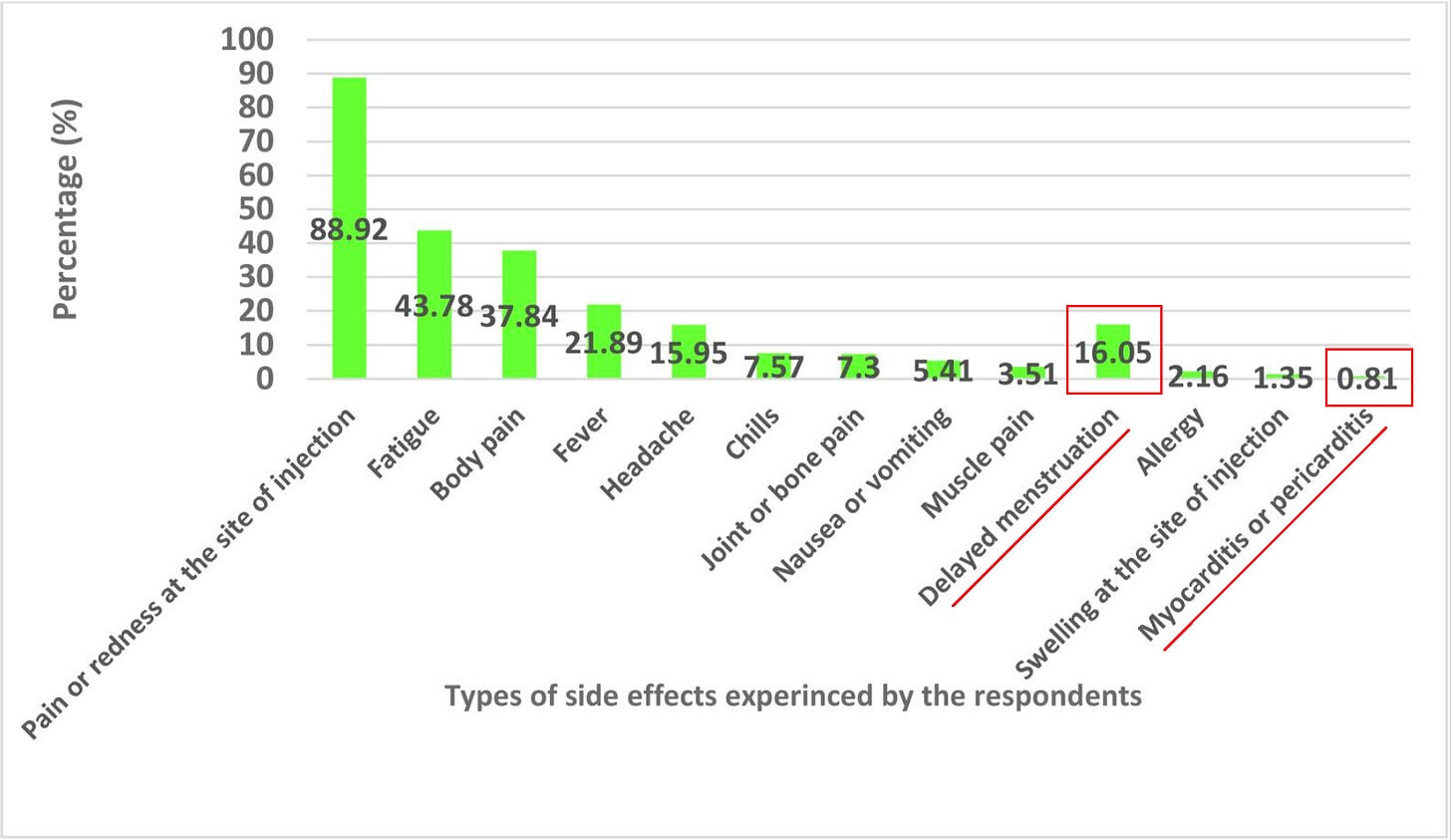
6. Prevalence of side-effects associated with the booster dose of Pfizer-BioNTech (BNT162b2) of COVID-19 Vaccine among vaccinated adults in the Eastern province of Saudi Arabia
https://pubmed.ncbi.nlm.nih.gov/36276167/
Pfizer booster safety profile (n = 370):
.82% reported myo/pericarditis (which would have to be clinically diagnosed)
16% reported delayed menstruation
1.1% of respondents were hospitalized
Conclusions
Our study showed that participants in the Eastern province of Saudi Arabia receiving a booster dose of the Pfizer-BioNTech (BNT162b2) COVID-19 vaccine commonly experienced short-lived side effects, especially pain or redness at the infection site, but also fatigue, body pain, fever and headache. 2.4% of recipients had sought medical attention for their side effects, although only four participants (1.1%) were admitted to the hospital after experiencing side effects. Findings from this study support the vaccine's safety as well as provide important baseline data for healthcare workers and the general public to be aware of possible side effects following the COVID-19 vaccine. In light of the current study, its results support the vaccine's safety and provide important baseline data that will raise healthcare workers' and the general public's awareness of COVID-19 vaccine expected side-effects. In this way, vaccine-hesitant individuals and pessimists might be convinced to accept the COVID-19 booster dose.
Worth repeating:
“Findings from this study support the vaccine's safety”
“In this way, vaccine-hesitant individuals and pessimists might be convinced to accept the COVID-19 booster dose”
7. Cognizance, adverse effects and motivation regarding COVID-19 vaccination amongst health care professionals: A cross-sectional study
https://dmp.umw.edu.pl/pdf/2022/59/1/13.pdf
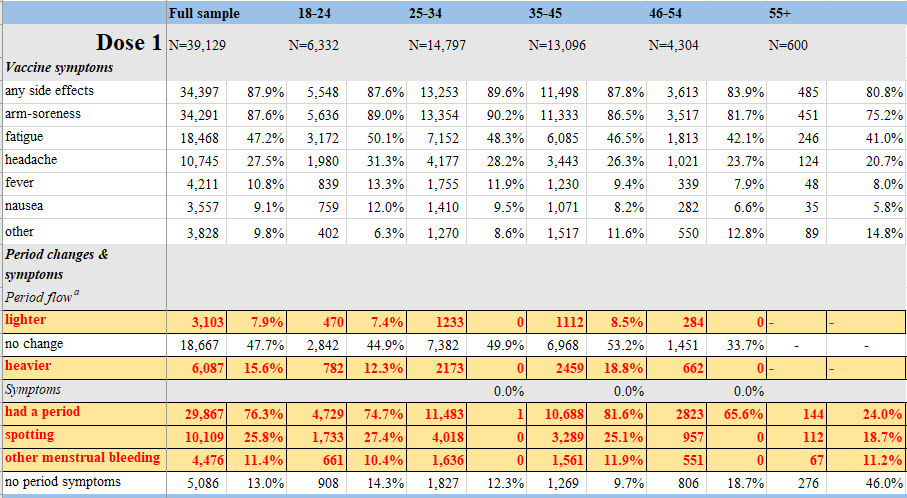
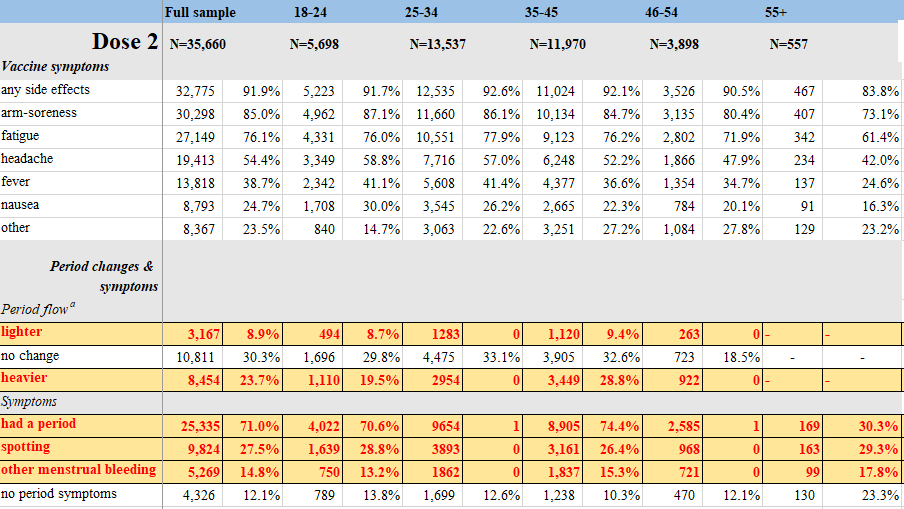
8. Investigating trends in those who experience menstrual bleeding changes after SARS-CoV-2 vaccination
https://www.medrxiv.org/content/10.1101/2021.10.11.21264863v2
Abstract
Early in 2021, many people began sharing that they experienced unexpected menstrual bleeding after SARS-CoV-2 inoculation. We investigated this emerging phenomenon of changed menstrual bleeding patterns among a convenience sample of currently and formerly menstruating people using a web-based survey. In this sample, 42% of people with regular menstrual cycles bled more heavily than usual while 44% reported no change after being vaccinated. Among respondents who typically do not menstruate, 71% of people on long-acting reversible contraceptives, 39% of people on gender-affirming hormones, and 66% of post-menopausal people reported breakthrough bleeding. We found increased/breakthrough bleeding was significantly associated with age, systemic vaccine side effects (fever, fatigue), history of pregnancy or birth, and ethnicity. Generally, changes to menstrual bleeding are not uncommon nor dangerous, yet attention to these experiences is necessary to build trust in medicine.
9. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8206586/
A total of 113 respondents completed the survey. Two doses of Pfizer/BioNTech vaccine (0.3 mL containing 30 micrograms of SARS-CoV-2 single-stranded, 5’-capped messenger RNA) were administered at distance of 21 days in the deltoid muscle of the upper arm.
Prevalence of women and current smokers was 73% and 15%, respectively. Mean age was 43±11 years. Prevalence of hypertension, diabetes mellitus, dyslipidemia, and asthma was 18%, 1%, 19%, and 10%, respectively. A previous myocardial infarction was recorded in 1 subject and 13 subjects had a previous documented exposure to SARS-CoV-2.
Adverse reactions occurred in 87% of subjects after the first dose and 83% after the second dose of vaccine. Most reactions were mild and none of the subjects discontinued normal daily activities. There was transitory loss of taste and smell in 1 subject. Body temperature >38.0 °C, tachycardia, or a rise in BP occurred in 13% and 27% of subjects after the first and second dose, respectively.
The subjects with documented infection by SARS-COV-2 over the previous year showed a higher frequency of systemic reactions to vaccine when compared with those without history of documented infection (38% vs 10%, p = 0.004).
Overall, 6 subjects (5.3%) showed an average rise in systolic or diastolic BP at home by ≥ 10 mmHg during the first 5 days after the first dose of vaccine when compared with the five days before the vaccine. Table 1 shows the main characteristics of these subjects. The BP rise required an intensification of BP-lowering treatment in 4 subjects. Two of the subjects with a BP rise after the first dose experienced a BP rise also after the second dose. Of note, history of COVID-19 was associated with a higher incidence of rise in BP when compared with subjects without previous exposure to SARS-CoV-2 (23% vs 3%, p = 0.002).
10. Israel MOH Booster Survey
Hebrew: https://drive.google.com/file/d/1NyMrHRTO-SLvygWtPmA39QlgqCE_GbsP/view
English: https://t.me/MOHreport/9197
11. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan
https://pubmed.ncbi.nlm.nih.gov/35058126/
12. Adverse Events Following COVID-19 Vaccination in Rivers State, Nigeria: A Cross-Sectional Study
https://pubmed.ncbi.nlm.nih.gov/35488575/
Results: In this study, 50.5% of respondents reported post-vaccination adverse events out of which 10 (4.6%) were severe (30% of the severe cases were life-threatening, 60% were hospitalised and 10% were placed on bed rest). The most common side effects were fever (73.0%), pain at the injection site (41.2%), fatigue (33.3%), body ache (17.5%) and headache (13.8%).
13. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea
https://pubmed.ncbi.nlm.nih.gov/33942579/
14. Side effects of the Pfizer BioNTech vaccine in health workers of a hospital in the southeast of Mexico
https://pubmed.ncbi.nlm.nih.gov/36223615/
15. Prevalence of severe adverse events among health professionals after receiving the first dose of the ChAdOx1 nCoV-19 coronavirus vaccine (Covishield) in Togo, March 2021
https://pubmed.ncbi.nlm.nih.gov/34819146/
Results
A total of 1,639 health professionals (70.2% male) with a median age of 32 (interquartile range: 27-40) were enrolled. At least one adverse event was reported among 71.6% of participants (95% CI = [69.3-73.8]). The most commonly reported adverse events were injection site pain (91.0%), asthenia (74.3%), headache (68.7%), soreness (55.0%), and fever (47.5%). An increased libido was also reported in 3.0% of participants. Of the participants who experienced adverse events, 18.2% were unable to go to work the day after vaccination, 10.5% consulted a medical doctor, and 1.0% were hospitalized. The SAEs’ prevalence was 23.8% (95% CI = [21.8-25.9]). Being <30 years (AOR = 5.54; p<0.001), or 30-49 years (AOR = 3.62; p<0.001) and being female (AOR = 1.97; p<0.001) were associated with SAEs.
In other words, as a % of the total number of participants:
13% were unable to go to work the next day
7.52% went to the doctor
0.72% were hospitalized
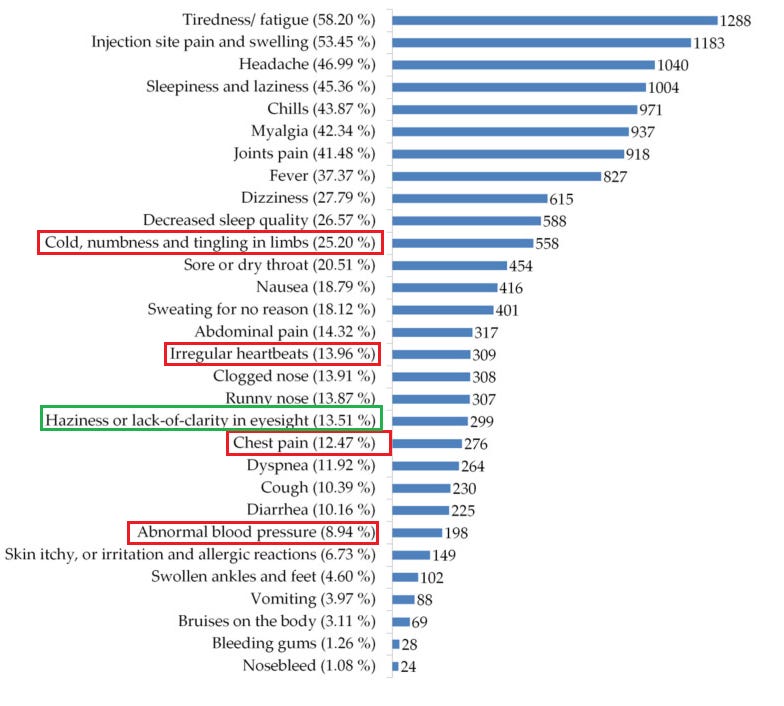
16. Determining the Health Problems Experienced by Young Adults in Turkey, Who Received the COVID-19 Vaccine
https://pubmed.ncbi.nlm.nih.gov/36146604/
I wrote up a partial explanation of this study’s findings here.
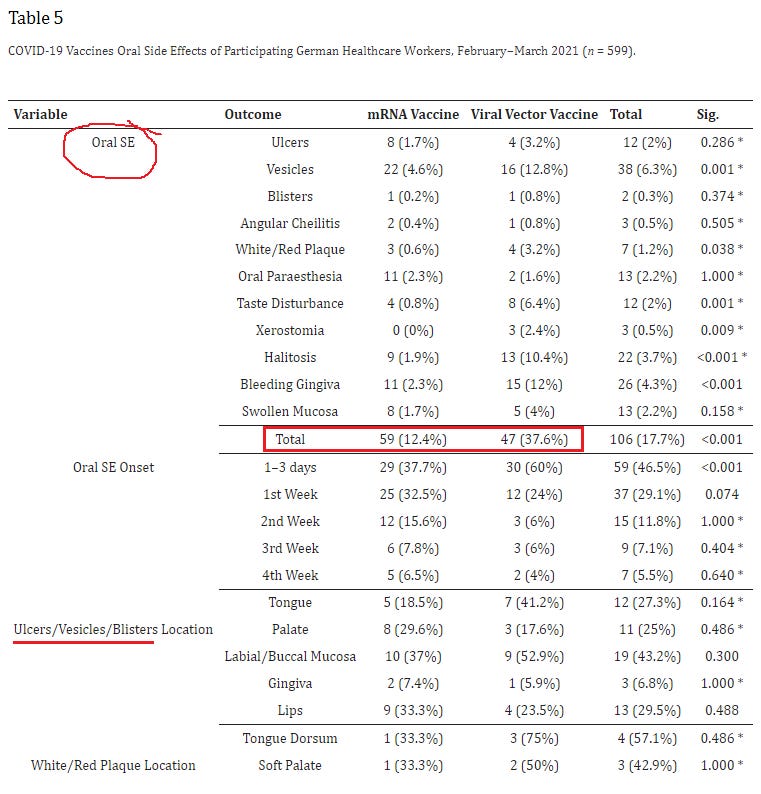
17. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers
https://pubmed.ncbi.nlm.nih.gov/34439984/
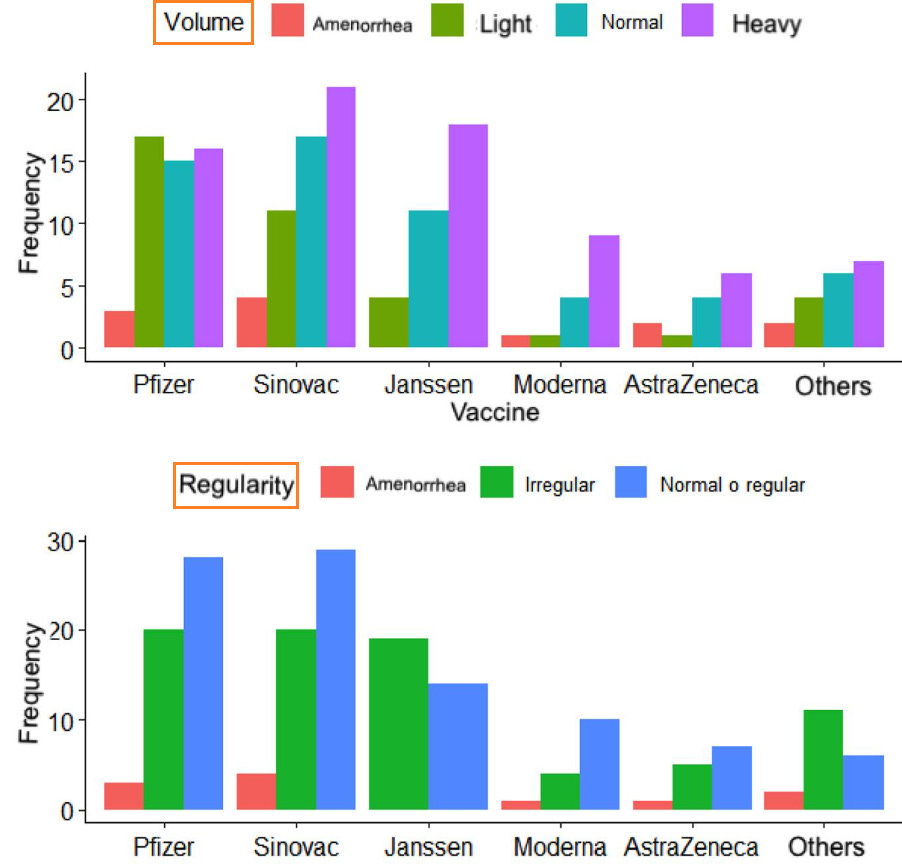
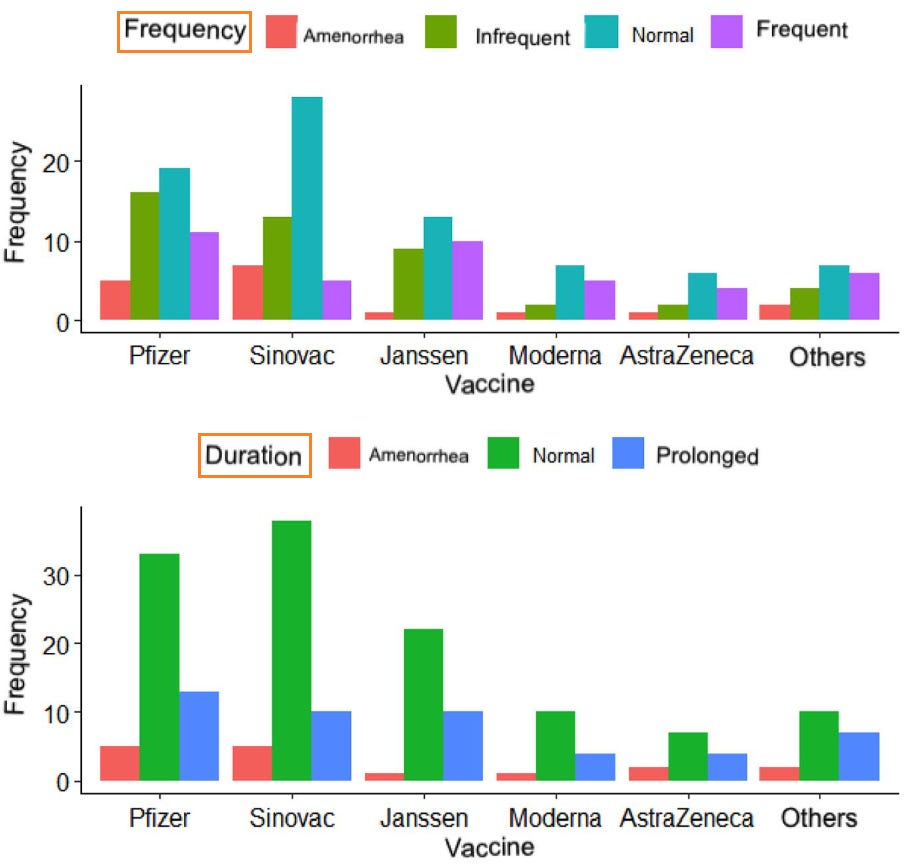
18. Menstrual cycle disturbances after COVID-19 vaccination
https://pubmed.ncbi.nlm.nih.gov/35796571/
Post-vaccination changes: regularity
Overall, 94 women (51.08%) reported regular cycles after vaccination, 79 (42.93%) noted irregular cycles after vaccination (variation > 9 days), and 11 (5.97%) of them reported having amenorrhea after vaccination. The vaccine that most frequently affected cycle regularity was Pfizer and Sinovac; however, for the J&J/Janssen vaccine more women reported irregular cycles (n = 19) than normal (58% versus 42%).
Post-vaccination changes: duration
In relation to the duration of the menstrual cycle, 120 women (65.21%) mentioned a duration in normal ranges (<8 days), 26.08% (48) commented a prolongation of the cycle (>9 days), and only 16 women (8.69%) reported amenorrhea/absent.
Post-vaccination changes: volume
The volume variable was reported as abnormal by 127 women (69.02%), being heavy in 41.84% (n = 77), light in 20.65% (n = 38), and absent in 6.52% (n = 12). Overall, 30.97% (n = 57) of the women described the volume as normal. When discriminating by biologics, 17 women (9.23%) vaccinated with Pfizer reported light volume, while the others reported predominantly heavy cycles (J&J/Janssen n = 18, Sinovac n = 21, Moderna n = 9, others n = 7, AstraZeneca n = 6).
Affectation in the quality of life
Of the 950 women who answered the survey, 31.36% (n = 298) commented that their QoL was affected after vaccination. Likewise, of the 184 who reported alterations in their menstrual cycle, 55.97% (103) stated a deterioration in their QoL after having been vaccinated in all the standard indicators evaluated perception of wealthiness, physical and mental health, pain.
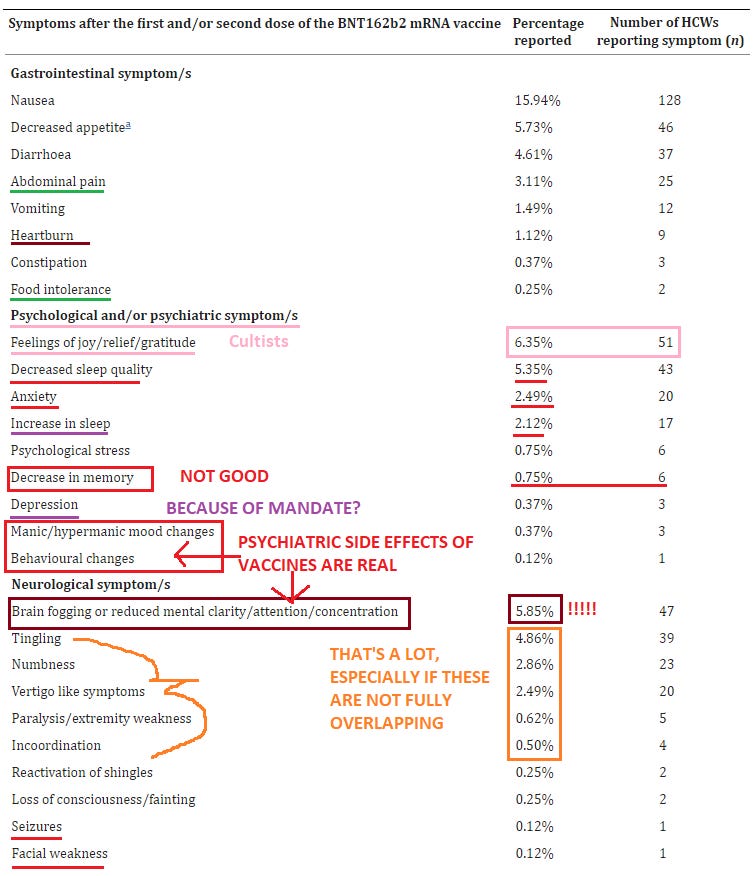
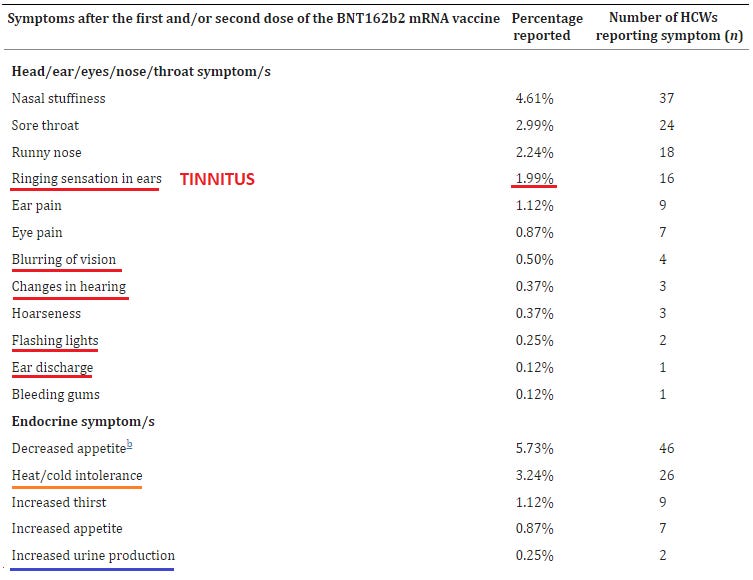
19. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers
https://pubmed.ncbi.nlm.nih.gov/33866000/
It’s amazing what you can find out when you just ask - this is an impressive amount of individual side effects that you won’t find in almost any other survey or study of vaccine ‘safety’.
Furthermore, this is a survey of healthcare workers, who are better trained to be observant of, & be able to describe with technical precision, clinical changes or side effects that occur, lending more credence than merely surveying a bunch of random laypeople who are more easily confused, susceptible to suggestion, and less precise in their observations and recall of clinical symptoms.
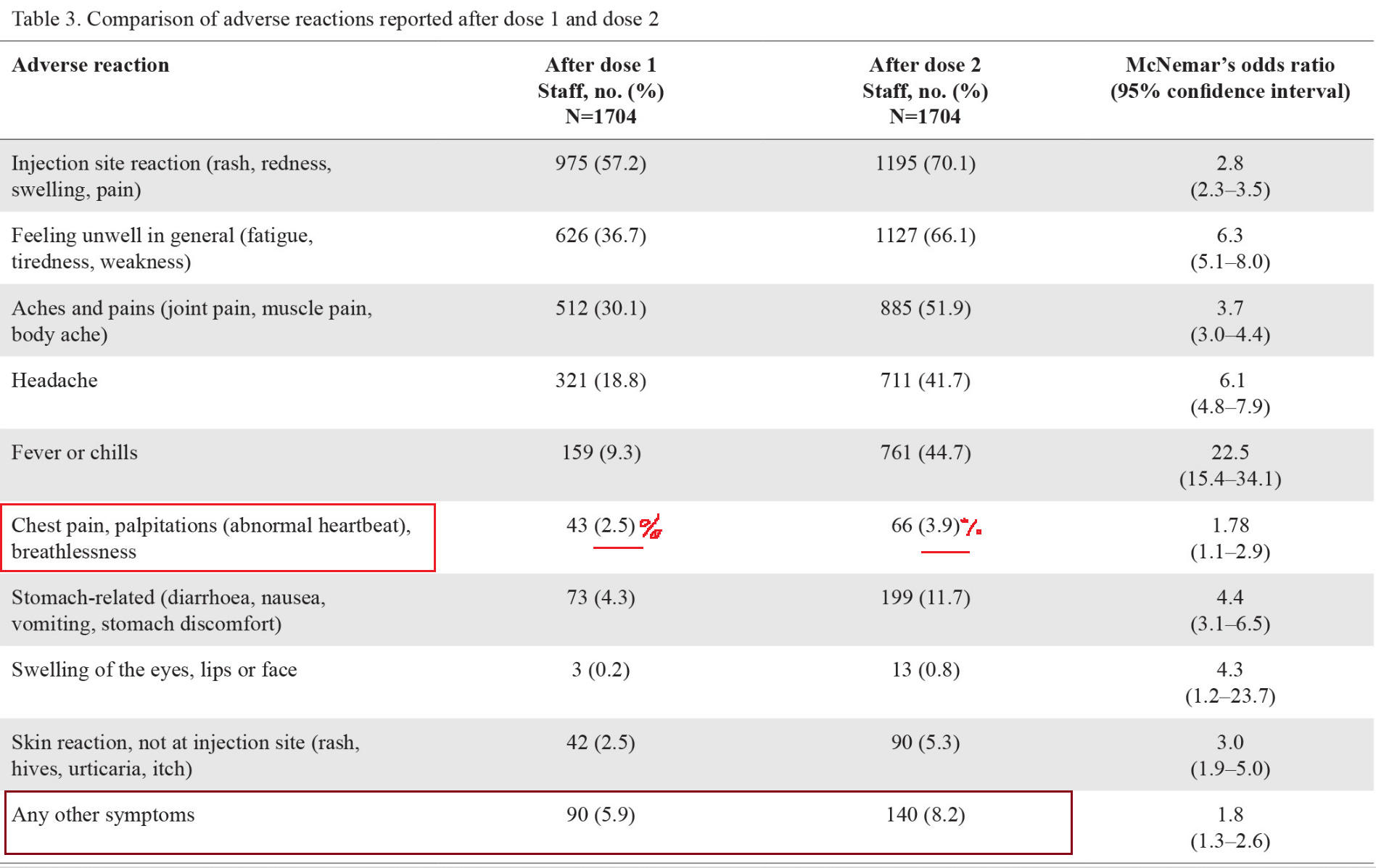
20. Safety and side effect profile of Pfizer-BioNTech COVID-19 vaccination among healthcare workers: A tertiary hospital experience in Singapore
https://pubmed.ncbi.nlm.nih.gov/34625758/












It is more than obvious that the mRNA gene altering injections do nothing to prevent covid, or stop the transmission of covid or provide any other health benefit. So what is their true purpose? I would guess some of those answers are provided above in the outstanding data sets provided. I think as we go forward month by month, then year by year, we are going to be missing more and more real data. The medical mafia is trying its darnedest to hide the horrors of these poisons.
hey, it's all worth it, to TPTB...viz, zombies.substack.com/p/anal-swabs-for-collecting-data-the/
ANAL SWABS FOR COLLECTiNG DATA THE NEW CURRENCY OF THE WORLD
BECAUSE THEY CAN'T WAiT FOR YOU TO POOP iT OUT
ART GALLERY
You think I’m joking? They aren’t! They want the data.
They’re coming for the data, inside your butt and can not wait for you to poop it out, the must have early access and will collect it by all means possible to feed the beast AI Artificial Intelligence.
bitchute.com/video/eJ3yG62GpCaT/